

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50086062 5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-carboxylic acid (3-bromo-5-pyridin-3-yl-phenyl)-amide::CHEMBL14368::TCMDC-139024
SMILES: COc1cc2CCN(C(=O)Nc3cc(Br)cc(c3)-c3cccnc3)c2cc1C(F)(F)F
InChI Key: InChIKey=FNWNLUXEHHLLML-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50086062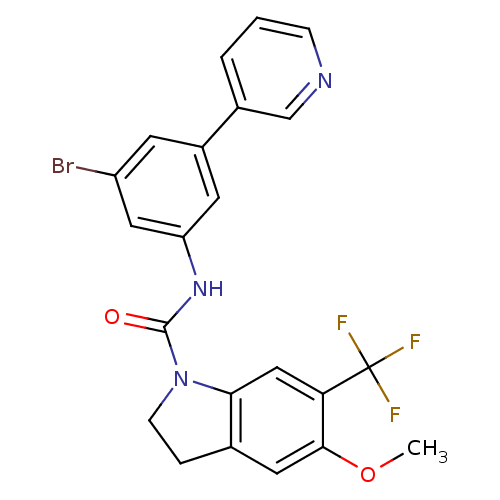 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. | J Med Chem 43: 1123-34 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q23X85V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50086062 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinities towards human cloned 5-hydroxytryptamine 2B receptor in HEK293 cells using [3H]5-HT as radioligand. | J Med Chem 43: 1123-34 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q23X85V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50086062 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine receptor 2A in HEK293 cells, using [3H]ketanserin as radioligand. | J Med Chem 43: 1123-34 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q23X85V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50086062 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human 5HT2c receptor expressed in CHO-K1 cells assessed as inhibition of 5HT-induced calcium influx measured up to 30 secs by aequorin ... | ACS Med Chem Lett 3: 373-377 (2012) Article DOI: 10.1021/ml300008j BindingDB Entry DOI: 10.7270/Q2PZ59X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50086062 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | PDB MMDB Reactome pathway KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of heterologously expressed human cytochrome P450 1A2. | J Med Chem 43: 1123-34 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q23X85V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||