

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50086434 CHEMBL3426035
SMILES: COCCO[C@H]1CC[C@@H](CC1)Nc1cc(=O)n(C)c2c(C)cc(cc12)-c1cncs1
InChI Key: InChIKey=OQXFWZCVLGUTPB-IYARVYRRSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymphocyte differentiation antigen CD38 (Homo sapiens (Human)) | BDBM50086434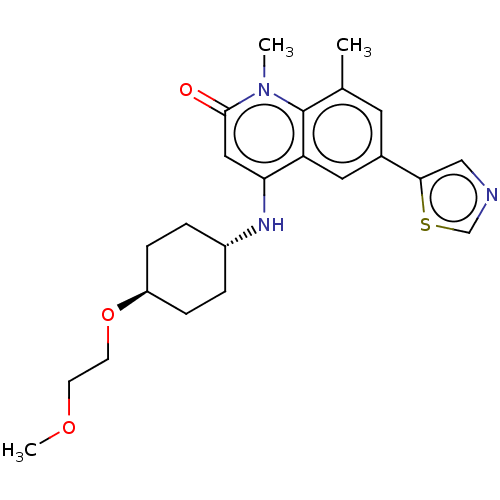 (CHEMBL3426035) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis | J Med Chem 58: 3548-71 (2015) Article DOI: 10.1021/jm502009h BindingDB Entry DOI: 10.7270/Q2NV9M00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 (Mus musculus) | BDBM50086434 (CHEMBL3426035) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of mouse recombinant CD38 extracellular domain expressed in CHO CGE cells assessed as NAD hydrolysis by fluorescence plate reader analysis | J Med Chem 58: 3548-71 (2015) Article DOI: 10.1021/jm502009h BindingDB Entry DOI: 10.7270/Q2NV9M00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphocyte differentiation antigen CD38 (Homo sapiens (Human)) | BDBM50086434 (CHEMBL3426035) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human CD38 extracellular domain expressed in Pichia pastoris assessed as NAD hydrolysis by colorimetric-based assay | J Med Chem 58: 3548-71 (2015) Article DOI: 10.1021/jm502009h BindingDB Entry DOI: 10.7270/Q2NV9M00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||