

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50087029 (1S,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexahydro-6-aza-benzo[mno]aceanthrylene::(6S,10aR)-6-methoxy-1,6-dimethyl-1,2,3,6,10,10a-hexahydrofluoreno[3,4,5-defg]quinoline::1-Methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexahydro-6-aza-benzo[mno]aceanthrylene::CHEMBL281923
SMILES: CO[C@@]1(C)c2cccc3C[C@H]4N(C)CCc5ccc1c(-c23)c45
InChI Key: InChIKey=JVZCJQBRRXZMQR-UZLBHIALSA-N
Data: 8 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serotonin (5-HT) receptor (Rattus norvegicus (rat)) | BDBM50087029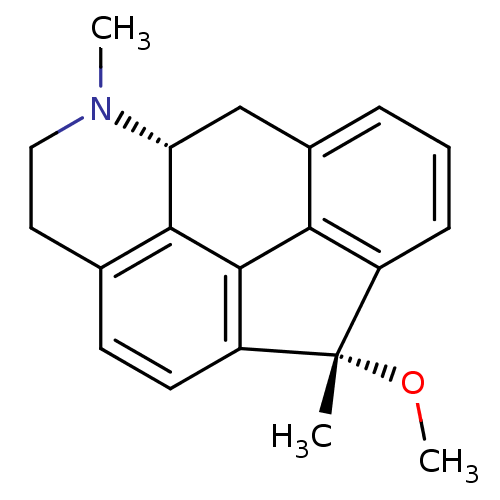 ((1S,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]-5-HT from over-expressed rat 5-hydroxytryptamine 7 receptor | J Med Chem 43: 1339-49 (2001) BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50087029 ((1S,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]5-HT from rat SERT | J Med Chem 50: 171-81 (2007) Article DOI: 10.1021/jm060959i BindingDB Entry DOI: 10.7270/Q22J6CPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50087029 ((1S,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 7 receptor | J Med Chem 46: 2795-812 (2003) Article DOI: 10.1021/jm030030n BindingDB Entry DOI: 10.7270/Q2M0465F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50087029 ((1S,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity was measured on cloned human 5-hydroxytryptamine 1A receptor which is labeled by [3H]-8-OH-DPAT | J Med Chem 43: 1339-49 (2001) BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50087029 ((1S,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat 5HT1A receptor | J Med Chem 50: 171-81 (2007) Article DOI: 10.1021/jm060959i BindingDB Entry DOI: 10.7270/Q22J6CPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50087029 ((1S,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Displacement of [3H]raclopride from rat dopamine D2 receptor | J Med Chem 50: 171-81 (2007) Article DOI: 10.1021/jm060959i BindingDB Entry DOI: 10.7270/Q22J6CPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50087029 ((1S,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Displacement of [3H]-Raclopride from human Dopamine receptor D2A | J Med Chem 43: 1339-49 (2001) BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50087029 ((1S,5aR)-1-methoxy-1,6-dimethyl-1,5,5a,6,7,8-hexah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 | J Med Chem 46: 2795-812 (2003) Article DOI: 10.1021/jm030030n BindingDB Entry DOI: 10.7270/Q2M0465F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||