

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50087085 (2S)-2-[(2S)-3-{[(1S)-1-azaniumyl-2-phenylethyl](hydroxy)phosphoryl}-2-[(4-phenylphenyl)methyl]propanamido]propanoic acid::1-{[3-Biphenyl-4-yl-2-(1-carboxy-ethylcarbamoyl)-propyl]-hydroxy-phosphinoyl}-2-phenyl-ethyl-ammonium
SMILES: C[C@H](NC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)CP(O)(=O)[C@H]([NH3+])Cc1ccccc1)C(O)=O
InChI Key: InChIKey=HYHWKYMUCMZAME-OWAUWMPXSA-O
Data: 5 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087085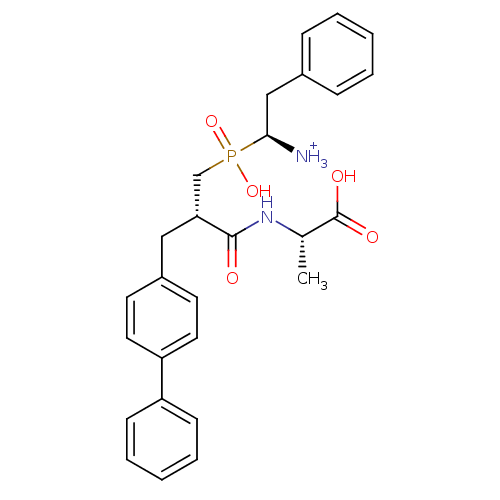 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumyl-2-phenylethyl](h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087085 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumyl-2-phenylethyl](h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087085 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumyl-2-phenylethyl](h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087085 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumyl-2-phenylethyl](h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50087085 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumyl-2-phenylethyl](h...) | KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description inhibitory activity on angiotensin I converting enzyme (ACE) using cbz-Phe-His-Leu as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||