

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50087690 8-Bicyclohexyl-4-yl-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one::CHEMBL354065
SMILES: O=C1NCN(c2ccccc2)C11CCN(CC1)[C@@H]1CC[C@@H](CC1)C1CCCCC1
InChI Key: InChIKey=PKXNDSOXWCRAIK-SZPZYZBQSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin/Orphanin FQ, NOP receptor (RAT) | BDBM50087690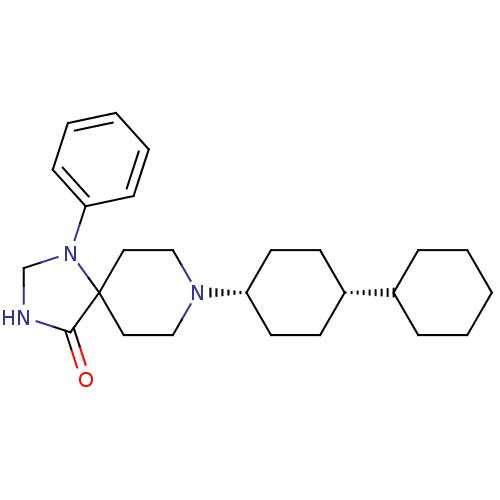 (8-Bicyclohexyl-4-yl-1-phenyl-1,3,8-triaza-spiro[4....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [leucyl-3H]-OFQ to membrane of human embryonic kidney 293 cells overexpressing rat Opioid receptor like 1 | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087690 (8-Bicyclohexyl-4-yl-1-phenyl-1,3,8-triaza-spiro[4....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50087690 (8-Bicyclohexyl-4-yl-1-phenyl-1,3,8-triaza-spiro[4....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [N-allyl-2-3-3H]-naloxone to membrane of baby hamster kidney cells infected with forest virus encoding the... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptors; mu & delta (Rattus norvegicus (rat)) | BDBM50087690 (8-Bicyclohexyl-4-yl-1-phenyl-1,3,8-triaza-spiro[4....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Inhibition against binding of radioligand [Ile5,6-3H]-deltorphin to membrane from baby hamster kidney cells infected with forest virus encoding the ... | Bioorg Med Chem Lett 10: 831-4 (2000) BindingDB Entry DOI: 10.7270/Q2N29XG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||