

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50088351 2-[2,15,17,29-tetrahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-13-methylcarbonyloxy-6,23-dioxo-8,30-dioxa-24-azatetracyclo[23.3.1.14,7.05,28]triaconta-1(28),2,4,9,19,21,25(29),26-octaen-27-yloxy]acetic acid::CHEMBL216962
SMILES: COC1\C=C\OC2(C)Oc3c(C2=O)c2c(O)c(C=NN4C(C)CN(Cc5ccc(cc5)[N+]([O-])=O)CC4C)c(NC(=O)\C(C)=C/C=C/C(C)C(O)C(C)C(O)C(C)C(OC(C)=O)C1C)c(O)c2c(O)c3C
InChI Key: InChIKey=GCIFILZADFJJJZ-NDQWUTJDSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50088351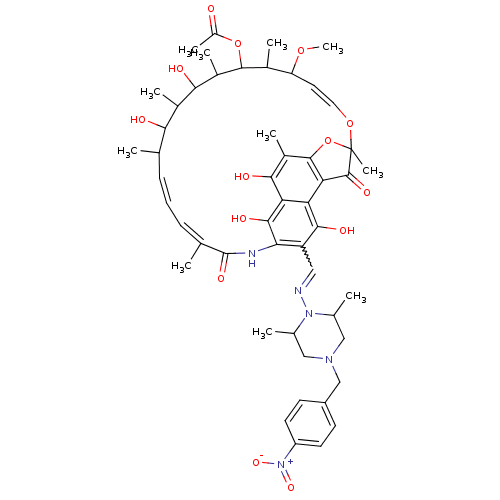 (2-[2,15,17,29-tetrahydroxy-11-methoxy-3,7,12,14,16...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of HIV-1 integrase, under 1 uM for the 3''-preprocessing | J Med Chem 43: 2100-14 (2000) BindingDB Entry DOI: 10.7270/Q27D2VTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50088351 (2-[2,15,17,29-tetrahydroxy-11-methoxy-3,7,12,14,16...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Tested for inhibition of HIV-1 integrase, under 1 uM for the strand transfer | J Med Chem 43: 2100-14 (2000) BindingDB Entry DOI: 10.7270/Q27D2VTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||