 Found 6 hits for monomerid = 50088360
Found 6 hits for monomerid = 50088360 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50088360
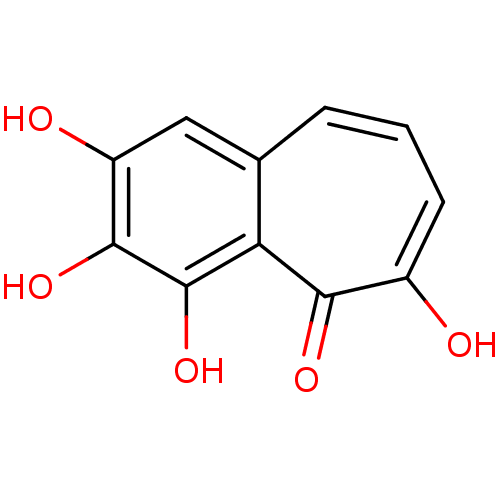
(2,3,4,6-tetrahydroxy-5H-benzo[7]annulen-5-one | 2,...)Show InChI InChI=1S/C11H8O5/c12-6-3-1-2-5-4-7(13)10(15)11(16)8(5)9(6)14/h1-4,13,15-16H,(H,12,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Moffitt Cancer Center and Research Institute
| Assay Description
The inhibitory against activated CDK2-cyclin A2 complex was determined by using the ADP Quest fluorescence assay from (DiscoveRX, Fremont, CA) |
Chembiochem 13: 2128-36 (2012)
Article DOI: 10.1002/cbic.201200316
BindingDB Entry DOI: 10.7270/Q2154FN2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
CDK2/Cyclin A/Cyclin A1
(Homo sapiens (Human)) | BDBM50088360

(2,3,4,6-tetrahydroxy-5H-benzo[7]annulen-5-one | 2,...)Show InChI InChI=1S/C11H8O5/c12-6-3-1-2-5-4-7(13)10(15)11(16)8(5)9(6)14/h1-4,13,15-16H,(H,12,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Competitive inhibition of human CDK2/cyclinA using PKTPKKAKKL as substrate in presence of ATP |
Bioorg Med Chem Lett 25: 3420-35 (2015)
BindingDB Entry DOI: 10.7270/Q2736SQ1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-synuclein
(Homo sapiens (Human)) | BDBM50088360

(2,3,4,6-tetrahydroxy-5H-benzo[7]annulen-5-one | 2,...)Show InChI InChI=1S/C11H8O5/c12-6-3-1-2-5-4-7(13)10(15)11(16)8(5)9(6)14/h1-4,13,15-16H,(H,12,14) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50088360

(2,3,4,6-tetrahydroxy-5H-benzo[7]annulen-5-one | 2,...)Show InChI InChI=1S/C11H8O5/c12-6-3-1-2-5-4-7(13)10(15)11(16)8(5)9(6)14/h1-4,13,15-16H,(H,12,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Bcl-xl |
J Med Chem 46: 4259-64 (2003)
Article DOI: 10.1021/jm030190z
BindingDB Entry DOI: 10.7270/Q2F47PWM |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50088360

(2,3,4,6-tetrahydroxy-5H-benzo[7]annulen-5-one | 2,...)Show InChI InChI=1S/C11H8O5/c12-6-3-1-2-5-4-7(13)10(15)11(16)8(5)9(6)14/h1-4,13,15-16H,(H,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO1 expressed in bacterial expression system using L-Tryptophan as substrate after 25 mins in absence of GSH and pre... |
Eur J Med Chem 126: 983-996 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.029
BindingDB Entry DOI: 10.7270/Q2RX9F9K |
More data for this
Ligand-Target Pair | |
Human immunodeficiency virus type 1 integrase
(Human immunodeficiency virus 1) | BDBM50088360

(2,3,4,6-tetrahydroxy-5H-benzo[7]annulen-5-one | 2,...)Show InChI InChI=1S/C11H8O5/c12-6-3-1-2-5-4-7(13)10(15)11(16)8(5)9(6)14/h1-4,13,15-16H,(H,12,14) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 integrase, under 1 uM for the 3''-preprocessing |
J Med Chem 43: 2100-14 (2000)
BindingDB Entry DOI: 10.7270/Q27D2VTS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data