

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50088528 CHEMBL3576921
SMILES: [H][C@@]1(C[C@H](O)[C@H](O)[C@@H](C)O1)O[C@H]1[C@H](C)O[C@@]([H])(O[C@H]2[C@@H](O)C[C@]([H])(O[C@@H]3[C@H](C)CCC[C@]4(C)C=C(C)[C@H](C)C[C@]44OC(=O)C(=C4O)C(=O)[C@@]4(CC)[C@]5([H])[C@@H](C)C(=O)C[C@H](O)[C@@]5([H])C=C(C)[C@@]4([H])\C(CC)=C/[C@H](CO)C[C@H]3C)O[C@@H]2C)[C@H](O)[C@@H]1OC
InChI Key: InChIKey=DRKBZEJUOBNVRH-TXWYELBGSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grp78 (Homo sapiens (Human)) | BDBM50088528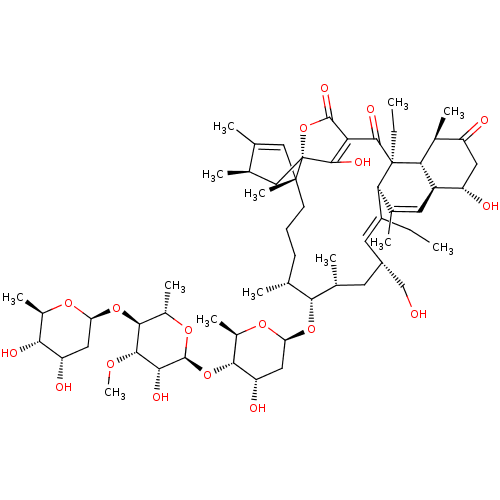 (CHEMBL3576921) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Diego Curated by ChEMBL | Assay Description Inhibition of 2-deoxyglucose-induced GRP78 (unknown origin) expression transfected in human HT1080 cells by luciferase reporter gene assay | J Nat Prod 78: 562-75 (2015) Article DOI: 10.1021/np500757w BindingDB Entry DOI: 10.7270/Q2HQ41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||