

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50089863 CHEMBL3577701
SMILES: COc1ccc2C(=O)CC(CC(=O)NCCNC(=O)c3ccccc3N3CCC(=O)NC3=O)c2c1
InChI Key: InChIKey=KRLQJSZEXYIYPQ-UHFFFAOYSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089863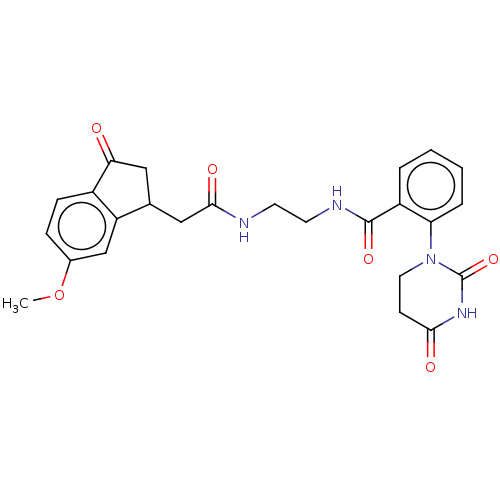 (CHEMBL3577701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50089863 (CHEMBL3577701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of tankyrase 2 catalytic domain (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089863 (CHEMBL3577701) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50089863 (CHEMBL3577701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of tankyrase 2 catalytic domain (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089863 (CHEMBL3577701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089863 (CHEMBL3577701) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||