

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50092157 (4R,5S)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxylic acid (4-bromo-phenyl)-amide::(4R,5S)-N-(4-bromophenyl)-3-oxo-4,5-diphenylpyrazolidine-1-carboxamide::3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxylic acid (4-bromo-phenyl)-amide(LY288512)::CHEMBL117281::LY-288512
SMILES: Brc1ccc(NC(=O)N2NC(=O)[C@@H]([C@H]2c2ccccc2)c2ccccc2)cc1
InChI Key: InChIKey=LMUQHXHWJWQXSD-WOJBJXKFSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin A receptor (Cavia porcellus) | BDBM50092157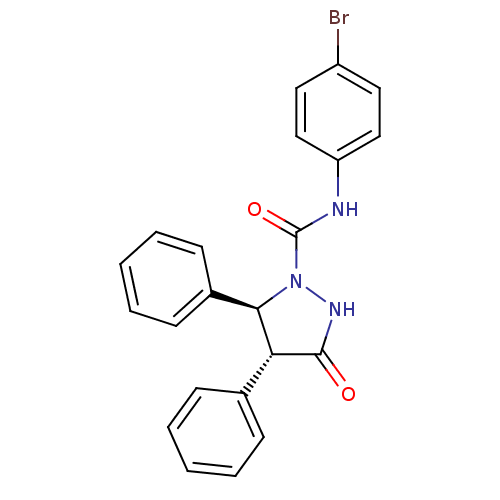 ((4R,5S)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxyl...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8S from CC1 receptor expressed in guinea pig pancreatic cells | J Med Chem 51: 565-73 (2008) Article DOI: 10.1021/jm070880t BindingDB Entry DOI: 10.7270/Q2TF014R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (MOUSE) | BDBM50092157 ((4R,5S)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 370 | n/a | n/a | n/a | n/a | 7.4 | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [125I]-CCK-8 to Cholecystokinin type B receptor in mouse brain at a pH of 6.5 | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (MOUSE) | BDBM50092157 ((4R,5S)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]Bolton-Hunter CCK-8 to Cholecystokinin type B receptor in the mouse cerebral cortex | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (RAT) | BDBM50092157 ((4R,5S)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its activity to inhibit the binding of [3H]-L-364,718 to Cholecystokinin type A receptor in rat pancreas | Bioorg Med Chem Lett 3: 875-880 (1993) Article DOI: 10.1016/S0960-894X(00)80684-8 BindingDB Entry DOI: 10.7270/Q21C1WTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor (RAT) | BDBM50092157 ((4R,5S)-3-Oxo-4,5-diphenyl-pyrazolidine-1-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Half maximal inhibition of specific binding of [125I]-Bolton-Hunter CCK-8 to Cholecystokinin type A receptor in the rat pancreas | J Med Chem 43: 3505-17 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9XBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||