

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50092244 5-[4-(4-Amino-phenoxy)-phenyl]-7-tert-butyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine::CHEMBL69068
SMILES: CC(C)(C)n1cc(-c2ccc(Oc3ccc(N)cc3)cc2)c2c(N)ncnc12
InChI Key: InChIKey=QJSJCFHSACXKCC-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50092244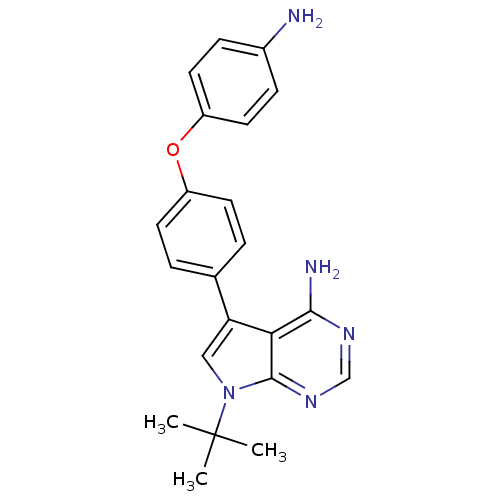 (5-[4-(4-Amino-phenoxy)-phenyl]-7-tert-butyl-7H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against tie-2 at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092244 (5-[4-(4-Amino-phenoxy)-phenyl]-7-tert-butyl-7H-pyr...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against p56 Lck tyrosine kinase at a concentration of 5 uM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50092244 (5-[4-(4-Amino-phenoxy)-phenyl]-7-tert-butyl-7H-pyr...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase (catalytic domain) | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50092244 (5-[4-(4-Amino-phenoxy)-phenyl]-7-tert-butyl-7H-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
BASF Bioresearch Corporation Curated by ChEMBL | Assay Description Inhibitory activity against vascular endothelial growth factor receptor 2 (VEGFR2) at a concentration of 5 microM ATP. | Bioorg Med Chem Lett 10: 2171-4 (2001) BindingDB Entry DOI: 10.7270/Q2DB813K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||