

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50092681 CHEMBL127434::N,N-Diethyl-4-[(3-methoxy-phenyl)-(2,5,5-trimethyl-piperazin-1-yl)-methyl]-benzamide
SMILES: CCN(CC)C(=O)c1ccc(cc1)C(N1CC(C)(C)NCC1C)c1cccc(OC)c1
InChI Key: InChIKey=HAILLLUQXQOSOC-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50092681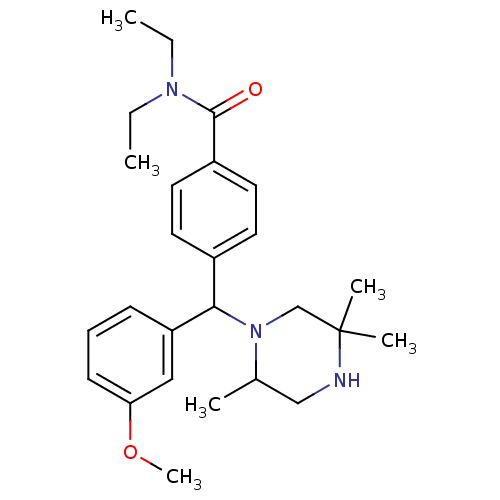 (CHEMBL127434 | N,N-Diethyl-4-[(3-methoxy-phenyl)-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal Curated by ChEMBL | Assay Description Agonist potency was measured using GTP gamma-[35S] binding assay | J Med Chem 43: 3878-94 (2000) BindingDB Entry DOI: 10.7270/Q2QN661W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50092681 (CHEMBL127434 | N,N-Diethyl-4-[(3-methoxy-phenyl)-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 using [125I]-D-Pro10-dynorphin A[1-11] as radioligand | J Med Chem 43: 3878-94 (2000) BindingDB Entry DOI: 10.7270/Q2QN661W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50092681 (CHEMBL127434 | N,N-Diethyl-4-[(3-methoxy-phenyl)-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor mu 1 using [125I]FK33824 as radioligand | J Med Chem 43: 3878-94 (2000) BindingDB Entry DOI: 10.7270/Q2QN661W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50092681 (CHEMBL127434 | N,N-Diethyl-4-[(3-methoxy-phenyl)-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal Curated by ChEMBL | Assay Description Binding affinity against human opioid receptor delta 1 using [125I]-[D-Ala2]-deltorphin II as radioligand | J Med Chem 43: 3878-94 (2000) BindingDB Entry DOI: 10.7270/Q2QN661W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||