

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50092826 (2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydroxy)amino]carbonyl}thio)methyl]-2-(carboxyamino)-2-oxoethyl]amino}-5-oxopentanoic acid::(S)-2-amino-5-((R)-3-((4-bromophenyl)hydroxycarbamoylthio)-1-(carboxymethylamino)-1-oxopropan-2-ylamino)-5-oxopentanoic acid::CHEMBL128872::S-(N-4bromophenyl-N-hydroxycarbamoyl)glutathione
SMILES: N[C@@H](CCC(=O)N[C@@H](CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC(O)=O)C(O)=O
InChI Key: InChIKey=IQPLOQWSCZRUCA-RYUDHWBXSA-N
Data: 7 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glyoxalase 1 (GLO1) (Homo sapiens (Human)) | BDBM50092826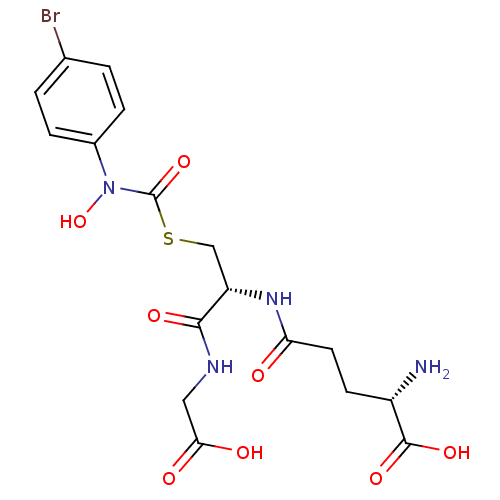 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human glyoxalase 1 | Bioorg Med Chem Lett 25: 4724-7 (2015) BindingDB Entry DOI: 10.7270/Q26H4K7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyoxalase I (Homo sapiens (Human)) | BDBM50092826 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chengdu University Curated by ChEMBL | Assay Description Inhibition of human erythrocyte Glyoxalase-1 | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115243 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyoxalase 1 (GLO1) (Homo sapiens (Human)) | BDBM50092826 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity for Glyoxalase I | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Saccharomyces cerevisiae) | BDBM50092826 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of yeast glyoxalase 1 | J Med Chem 52: 4650-6 (2009) Article DOI: 10.1021/jm900382u BindingDB Entry DOI: 10.7270/Q2S46SWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyoxalase 1 (GLO1) (Homo sapiens (Human)) | BDBM50092826 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound on yeast glyoxalase I (GlxI) was determined | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Saccharomyces cerevisiae) | BDBM50092826 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of yeast glyoxalase1 | Bioorg Med Chem Lett 16: 6039-42 (2006) Article DOI: 10.1016/j.bmcl.2006.08.121 BindingDB Entry DOI: 10.7270/Q2V987QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glyoxalase 1 (GLO1) (Homo sapiens (Human)) | BDBM50092826 ((2S)-2-amino-5-{[(1R)-1-[({[(4-bromophenyl)(hydrox...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Binding affinity of the compound on Pseudomonas putida glyoxalase I (GlxI) was determined | J Med Chem 43: 3981-6 (2000) BindingDB Entry DOI: 10.7270/Q2BC3XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||