

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50093011 1-((2S,4R)-1-((S)-2-((S)-2-acetamido-2-cyclohexylacetamido)-3-methylbutanoyl)-4-(naphthalen-1-ylmethoxy)pyrrolidine-2-carboxamido)cyclopropanecarboxylic acid::1-{[(R)-(S)-1-[(S)-2-((S)-2-Acetylamino-2-cyclohexyl-acetylamino)-3-methyl-butyryl]-4-(naphthalen-1-ylmethoxy)-pyrrolidine-2-carbonyl]-amino}-cyclopropanecarboxylic acid::1-{[1-[2-(2-Acetylamino-2-cyclohexyl-acetylamino)-3-methyl-butyryl]-4-(naphthalen-1-ylmethoxy)-pyrrolidine-2-carbonyl]-amino}-cyclopropanecarboxylic acid::CHEMBL75558
SMILES: CC(C)[C@H](NC(=O)[C@@H](NC(C)=O)C1CCCCC1)C(=O)N1C[C@@H](C[C@H]1C(=O)NC1(CC1)C(O)=O)OCc1cccc2ccccc12
InChI Key: InChIKey=ZTWLENQVRALUEE-ZRJFKUPHSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50093011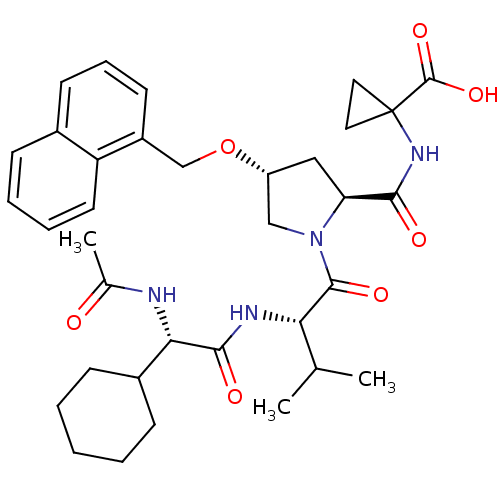 (1-((2S,4R)-1-((S)-2-((S)-2-acetamido-2-cyclohexyla...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibitory activity of the compound against hepatitis C virus NS3 protease | J Med Chem 47: 123-32 (2003) Article DOI: 10.1021/jm0303002 BindingDB Entry DOI: 10.7270/Q2Q52P2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50093011 (1-((2S,4R)-1-((S)-2-((S)-2-acetamido-2-cyclohexyla...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibitory activity against NS3 protease complexed with NS4A cofactor peptide (NS3-4A pep) | Bioorg Med Chem Lett 10: 2267-70 (2001) BindingDB Entry DOI: 10.7270/Q2S1831T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50093011 (1-((2S,4R)-1-((S)-2-((S)-2-acetamido-2-cyclohexyla...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3 protease | J Med Chem 47: 123-32 (2003) Article DOI: 10.1021/jm0303002 BindingDB Entry DOI: 10.7270/Q2Q52P2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50093011 (1-((2S,4R)-1-((S)-2-((S)-2-acetamido-2-cyclohexyla...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3 protease | J Med Chem 47: 123-32 (2003) Article DOI: 10.1021/jm0303002 BindingDB Entry DOI: 10.7270/Q2Q52P2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50093011 (1-((2S,4R)-1-((S)-2-((S)-2-acetamido-2-cyclohexyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human leukocyte elastase (HLE) | Bioorg Med Chem Lett 10: 2267-70 (2001) BindingDB Entry DOI: 10.7270/Q2S1831T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||