

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50093523 CHEMBL3585679::PTP1B spring 6 (6)
SMILES: OCc1ccc(-c2ccc(O)cc2)c(C(O)=O)c1C#Cc1ccc(O)cc1
InChI Key: InChIKey=VBLCSEMPFRLYCH-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50093523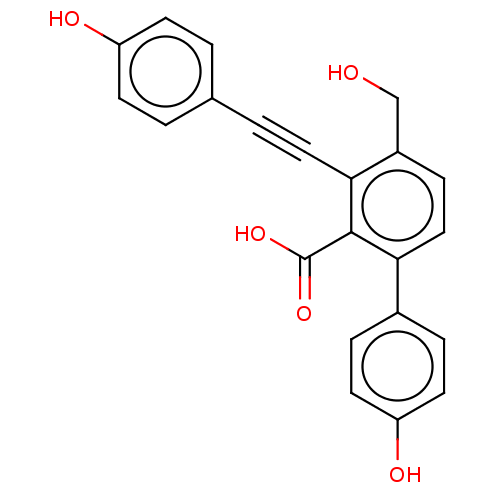 (CHEMBL3585679 | PTP1B spring 6 (6)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | 1.45E+4 | -6.86 | 1.32E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50093523 (CHEMBL3585679 | PTP1B spring 6 (6)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured after 20 mins by micr... | Bioorg Med Chem 23: 3730-7 (2015) Article DOI: 10.1016/j.bmc.2015.04.007 BindingDB Entry DOI: 10.7270/Q25T3N81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50093523 (CHEMBL3585679 | PTP1B spring 6 (6)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of recombinant human MMP9 preincubated for 30 mins followed by McaPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate addition measured by fluoresce... | Bioorg Med Chem Lett 28: 2413-2417 (2018) Article DOI: 10.1016/j.bmcl.2018.06.024 BindingDB Entry DOI: 10.7270/Q2348P07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50093523 (CHEMBL3585679 | PTP1B spring 6 (6)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of recombinant human MMP2 preincubated for 30 mins followed by McaPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate addition measured by fluoresce... | Bioorg Med Chem Lett 28: 2413-2417 (2018) Article DOI: 10.1016/j.bmcl.2018.06.024 BindingDB Entry DOI: 10.7270/Q2348P07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase (2 and 3) (Homo sapiens (Human)) | BDBM50093523 (CHEMBL3585679 | PTP1B spring 6 (6)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of recombinant human MMP3 preincubated for 30 mins followed by McaPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate addition measured by fluoresce... | Bioorg Med Chem Lett 28: 2413-2417 (2018) Article DOI: 10.1016/j.bmcl.2018.06.024 BindingDB Entry DOI: 10.7270/Q2348P07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine phosphatase 1B (Homo sapiens (Human)) | BDBM50093523 (CHEMBL3585679 | PTP1B spring 6 (6)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Catholic University of Daegu Curated by ChEMBL | Assay Description Mixed-competitive inhibition of human recombinant PTP1B using p-NPP as substrate preincubated for 10 mins followed by substrate addition measured aft... | Bioorg Med Chem 23: 3730-7 (2015) Article DOI: 10.1016/j.bmc.2015.04.007 BindingDB Entry DOI: 10.7270/Q25T3N81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||