

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50095520 (S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1(6H)-yl)-N-(1-(5-tert-butyl-1,3,4-oxadiazol-2-yl)-3-methyl-1-oxobutan-2-yl)acetamide::(S)-2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-1-yl]-N-[1-(5-tert-butyl-[1,3,4]oxadiazole-2-carbonyl)-2-methyl-propyl]-acetamide::2-[5-Amino-2-(4-fluoro-phenyl)-6-oxo-6H-pyrimidin-1-yl]-N-[1-(5-tert-butyl-[1,3,4]oxadiazole-2-carbonyl)-2-methyl-propyl]-acetamide::CHEMBL282705
SMILES: CC(C)[C@H](NC(=O)Cn1c(ncc(N)c1=O)-c1ccc(F)cc1)C(=O)c1nnc(o1)C(C)(C)C
InChI Key: InChIKey=BFQPYKDOXQIVNB-KRWDZBQOSA-N
Data: 3 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50095520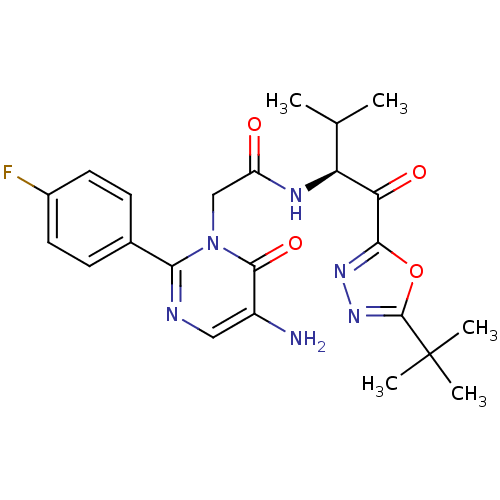 ((S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50095520 ((S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50095520 ((S)-2-(5-amino-2-(4-fluorophenyl)-6-oxopyrimidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||