

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50096724 (S)-4-((S)-1-{(S)-1-[(S)-1-((R)-1-Aminooxalyl-pentylcarbamoyl)-3-methyl-butylcarbamoyl]-2,2-dimethyl-propylcarbamoyl}-2-o-tolyl-ethylcarbamoyl)-4-[(S)-3-carboxy-2-(3-carboxy-propionylamino)-propionylamino]-butyric acid::CHEMBL331243
SMILES: CCCC[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)C(N)=O
InChI Key: InChIKey=CEEARFKAZMLMSC-NOCPHRDISA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50096724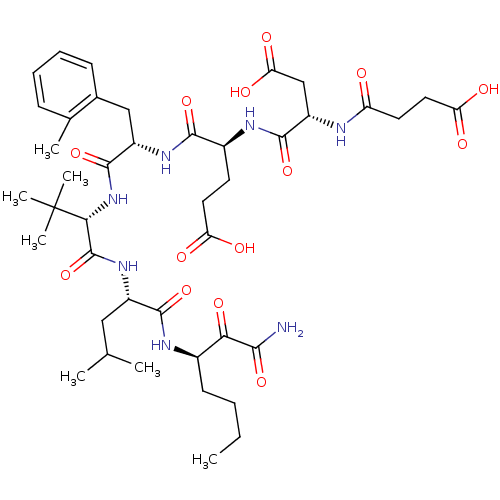 ((S)-4-((S)-1-{(S)-1-[(S)-1-((R)-1-Aminooxalyl-pent...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn Curated by ChEMBL | Assay Description Compound was tested for inhibition of Elastase | Bioorg Med Chem Lett 11: 355-7 (2001) BindingDB Entry DOI: 10.7270/Q2WQ0322 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis C virus serine protease, NS3/NS4A (Hepatitis C virus) | BDBM50096724 ((S)-4-((S)-1-{(S)-1-[(S)-1-((R)-1-Aminooxalyl-pent...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 variant with Y181C plus K103N mutatio... | Bioorg Med Chem Lett 11: 355-7 (2001) BindingDB Entry DOI: 10.7270/Q2WQ0322 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Homo sapiens (Human)) | BDBM50096724 ((S)-4-((S)-1-{(S)-1-[(S)-1-((R)-1-Aminooxalyl-pent...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn Curated by ChEMBL | Assay Description Inhibitory activity against Opioid receptor mu 1 of (endomorphin 2) in guinea pig ileum was determined | Bioorg Med Chem Lett 11: 355-7 (2001) BindingDB Entry DOI: 10.7270/Q2WQ0322 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin (Homo sapiens (Human)) | BDBM50096724 ((S)-4-((S)-1-{(S)-1-[(S)-1-((R)-1-Aminooxalyl-pent...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn Curated by ChEMBL | Assay Description Compound was tested for inhibition of Serine protease chymotrypsin | Bioorg Med Chem Lett 11: 355-7 (2001) BindingDB Entry DOI: 10.7270/Q2WQ0322 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||