

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50099258 6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-naphthalen-2-yl-1H-indole::CHEMBL297136
SMILES: F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccc2ccccc2c1
InChI Key: InChIKey=DAUSNPVLXKKGNQ-GFOWMXPYSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50099258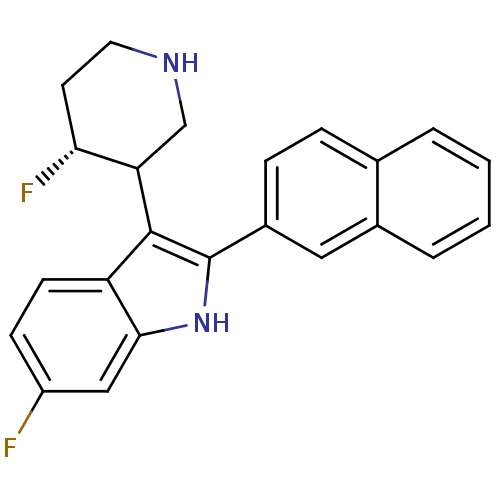 (6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-naphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Ability to displace [3H]-ketanserin binding to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 44: 1603-14 (2001) BindingDB Entry DOI: 10.7270/Q27M076K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50099258 (6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-naphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human ERG channel | J Med Chem 52: 4266-76 (2009) Article DOI: 10.1021/jm900002x BindingDB Entry DOI: 10.7270/Q2MK6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50099258 (6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-naphthalen-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Ability to displace [3H]-spiperone binding to CHO cells stably expressing dopamine receptor D2 | J Med Chem 44: 1603-14 (2001) BindingDB Entry DOI: 10.7270/Q27M076K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50099258 (6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-naphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from HEK cells expressing hERG voltage dependent IKr potassium channel Kv11.1 | J Med Chem 44: 1603-14 (2001) BindingDB Entry DOI: 10.7270/Q27M076K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||