

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50100962 1N-amino(immino)methyl-4-methylaniline::CHEMBL43415::N-(4-methylphenyl)guanidine
SMILES: [#6]-c1ccc(cc1)\[#7]=[#6](\[#7])-[#7]
InChI Key: InChIKey=PKQYVFHRKFDVCH-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50100962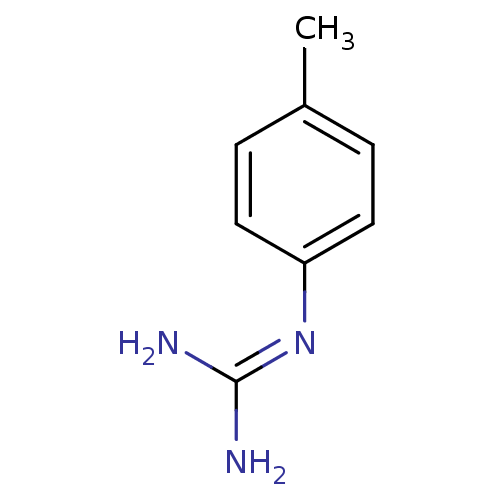 (1N-amino(immino)methyl-4-methylaniline | CHEMBL434...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition against Urokinase-type plasminogen activator | J Med Chem 33: 2956-61 (1990) BindingDB Entry DOI: 10.7270/Q2GB24NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator/surface receptor (Homo sapiens (Human)) | BDBM50100962 (1N-amino(immino)methyl-4-methylaniline | CHEMBL434...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition against Urokinase-type plasminogen activator | J Med Chem 33: 2956-61 (1990) BindingDB Entry DOI: 10.7270/Q2GB24NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin (Bos taurus (bovine)) | BDBM50100962 (1N-amino(immino)methyl-4-methylaniline | CHEMBL434...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition against Trypsin | J Med Chem 33: 2956-61 (1990) BindingDB Entry DOI: 10.7270/Q2GB24NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypsin I (Bos taurus (bovine)) | BDBM50100962 (1N-amino(immino)methyl-4-methylaniline | CHEMBL434...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition against Trypsin | J Med Chem 33: 2956-61 (1990) BindingDB Entry DOI: 10.7270/Q2GB24NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50100962 (1N-amino(immino)methyl-4-methylaniline | CHEMBL434...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | <1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition against human plasmin was determined at 0.5 mM | J Med Chem 33: 2956-61 (1990) BindingDB Entry DOI: 10.7270/Q2GB24NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 2 (Homo sapiens (Human)) | BDBM50100962 (1N-amino(immino)methyl-4-methylaniline | CHEMBL434...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutics, School of Pharmacy, Virginia Commonwealth University, Richmond, VA 23298, USA. Curated by ChEMBL | Assay Description Inhibition of human OCT2 expressed in HEK293 cells assessed as decrease in uptake of substrate [3H]MPP+ after 1 min by liquid scintillation counting ... | Bioorg Med Chem Lett 27: 4440-4445 (2017) Article DOI: 10.1016/j.bmcl.2017.08.008 BindingDB Entry DOI: 10.7270/Q24M970C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 3 (Homo sapiens (Human)) | BDBM50100962 (1N-amino(immino)methyl-4-methylaniline | CHEMBL434...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutics, School of Pharmacy, Virginia Commonwealth University, Richmond, VA 23298, USA. Curated by ChEMBL | Assay Description Inhibition of OCT3 (unknown origin) | Bioorg Med Chem Lett 27: 4440-4445 (2017) Article DOI: 10.1016/j.bmcl.2017.08.008 BindingDB Entry DOI: 10.7270/Q24M970C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 1 (Homo sapiens (Human)) | BDBM50100962 (1N-amino(immino)methyl-4-methylaniline | CHEMBL434...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutics, School of Pharmacy, Virginia Commonwealth University, Richmond, VA 23298, USA. Curated by ChEMBL | Assay Description Inhibition of human OCT1 expressed in HEK293 cells assessed as decrease in uptake of substrate [3H]MPP+ after 1 min by liquid scintillation counting ... | Bioorg Med Chem Lett 27: 4440-4445 (2017) Article DOI: 10.1016/j.bmcl.2017.08.008 BindingDB Entry DOI: 10.7270/Q24M970C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||