

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50101822 (Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-oct-1-enyl)-5-oxo-cyclopentyl]-hept-5-enoic acid::CHEMBL64804::PGE2, 15-epi::US9180116, PGE2
SMILES: CCCCC[C@@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O
InChI Key: InChIKey=XEYBRNLFEZDVAW-GKEZHNTDSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50101822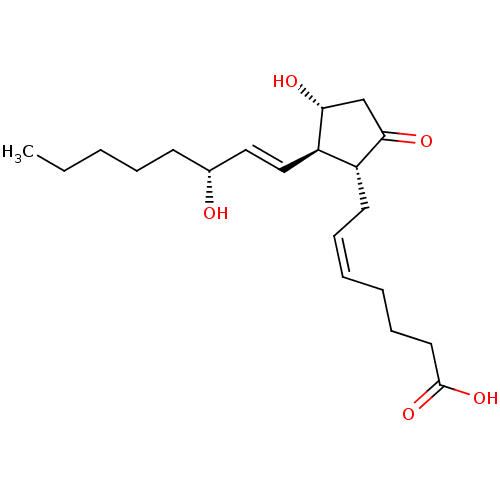 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | 0.380 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid EP4 Receptor (Mus musculus (Mouse)) | BDBM50101822 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP4 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid EP3 Receptor (Mus musculus (Mouse)) | BDBM50101822 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid EP1 Receptor (Mus musculus (Mouse)) | BDBM50101822 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP1 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid EP2 Receptor (Mus musculus (Mouse)) | BDBM50101822 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP2 receptor expressed in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid IP receptor (Homo sapiens (Human)) | BDBM50101822 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid IP receptor (Homo sapiens (Human)) | BDBM50101822 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Royal Postgraduate Medical School Curated by PDSP Ki Database | Br J Pharmacol 72: 435-41 (1981) Article DOI: 10.1111/j.1476-5381.1981.tb10994.x BindingDB Entry DOI: 10.7270/Q2HM56XZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| PTGIR (MOUSE) | BDBM50101822 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Royal Postgraduate Medical School Curated by PDSP Ki Database | Biochem Pharmacol 30: 2041-4 (1981) Article DOI: 10.1016/0006-2952(81)90220-3 BindingDB Entry DOI: 10.7270/Q2668BPP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid IP receptor (Homo sapiens (Human)) | BDBM50101822 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Effective concentration which increases intracellular c-AMP production in human Prostanoid IP receptor | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50101822 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | 37 |
Cayman Chemical Company, Inc. US Patent | Assay Description 1. Seed cells on an EP2 or EP4 STEP plate at a density of 40,000-80,000 cells/well in 200 ul of reduced serum medium containing 0.5% FBS. Place the p... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50101822 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 0.0500 | n/a | n/a | n/a | 37 |
Cayman Chemical Company, Inc. US Patent | Assay Description 1. Seed cells on an EP2 or EP4 STEP plate at a density of 40,000-80,000 cells/well in 200 ul of reduced serum medium containing 0.5% FBS. Place the p... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50101822 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 0.480 | n/a | n/a | n/a | 37 |
Cayman Chemical Company, Inc. US Patent | Assay Description 1. Seed cells on an EP2 or EP4 STEP plate at a density of 40,000-80,000 cells/well in 200 uL of reduced serum medium containing 0.5% FBS. Place the p... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostanoid EP2 receptor (Mus musculus (Mouse)) | BDBM50101822 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||