

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50102274 1-(5-Bromo-pyridin-2-yl)-3-[2-(7,8-dimethyl-pyrrolo[1,2-a]quinoxalin-4-yloxy)-ethyl]-thiourea::CHEMBL317406
SMILES: Cc1cc2nc(OCCNC(=S)Nc3ccc(Br)cn3)c3cccn3c2cc1C
InChI Key: InChIKey=KFMGHZLWRUNPSU-UHFFFAOYSA-N
Data: 7 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50102274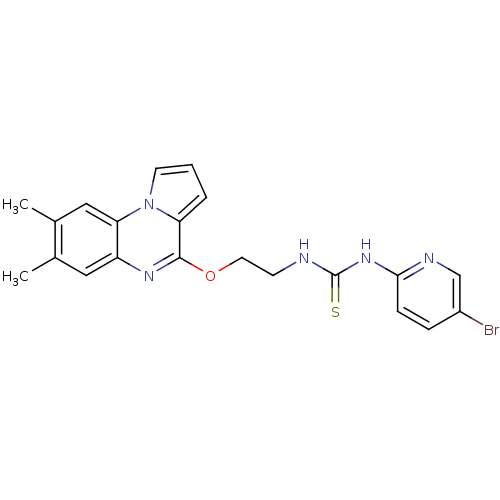 (1-(5-Bromo-pyridin-2-yl)-3-[2-(7,8-dimethyl-pyrrol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution V106A | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50102274 (1-(5-Bromo-pyridin-2-yl)-3-[2-(7,8-dimethyl-pyrrol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 wild-type RT | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50102274 (1-(5-Bromo-pyridin-2-yl)-3-[2-(7,8-dimethyl-pyrrol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution K103N | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50102274 (1-(5-Bromo-pyridin-2-yl)-3-[2-(7,8-dimethyl-pyrrol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution L1001 | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50102274 (1-(5-Bromo-pyridin-2-yl)-3-[2-(7,8-dimethyl-pyrrol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution Y181I | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50102274 (1-(5-Bromo-pyridin-2-yl)-3-[2-(7,8-dimethyl-pyrrol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution V179D | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50102274 (1-(5-Bromo-pyridin-2-yl)-3-[2-(7,8-dimethyl-pyrrol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Salerno Curated by ChEMBL | Assay Description Inhibition of HIV-1 Mutant HIV-1 RT enzymes containing the single amino acid substitution Y188L | J Med Chem 44: 305-15 (2001) BindingDB Entry DOI: 10.7270/Q2HX1DD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||