

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50102711 7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid {1-[4-(3-hydroxy-phenyl)-3,4-dimethyl-piperidin-1-ylmethyl]-2-methyl-propyl}-amide::CHEMBL51204
SMILES: CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)C1Cc2ccc(O)cc2CN1
InChI Key: InChIKey=ZLVXBBHTMQJRSX-HUUIFEGFSA-N
Data: 9 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50102711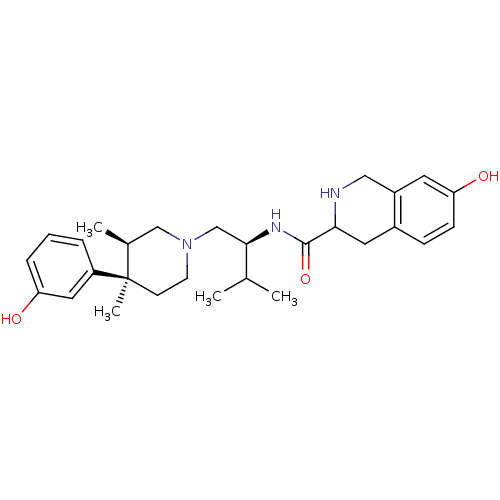 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist (U50,488) stimulated [35S]GTP-gamma-S, binding in cloned opioid receptor kappa 1 | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of stimulation of [35S]GTP-gamma-S, binding produced by the selective agonist (U69593, kappa-receptor), in guinea pig caudate membranes. | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity to opioid receptor kappa 1 of guinea pig brain, using [3H]U-69593 as radioligand | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of stimulation of [35S]GTP-gamma-S, binding produced by the selective agonist (DAMGO, mu-receptor), in guinea pig caudate membranes. | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist (DAMGO) stimulated [35S]GTP-gamma-S, binding in cloned mu opioid receptors. | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity to mu-opioid receptor of rat brain using [3H]DAMGO as radioligand. | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist (SNC-80) stimulated [35S]GTP-gamma-S, binding in cloned opioid receptor mu1 | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of stimulation of [35S]GTP-gamma-S, binding produced by the selective agonist (SNC-80, delta-receptor), in guinea pig caudate membranes | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptors; mu & delta (Rattus norvegicus (rat)) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 301 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity to delta-opioid receptor of rat brain using [3H]DADLE as radioligand | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||