 Found 13 hits for monomerid = 50103568
Found 13 hits for monomerid = 50103568 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lactate dehydrogenase A (LDHA)
(Homo sapiens (Human)) | BDBM50103568
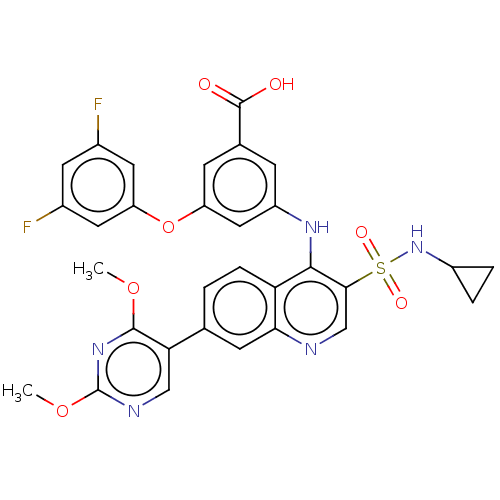
(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of LDHA (unknown origin) |
Bioorg Med Chem Lett 24: 4915-25 (2014)
BindingDB Entry DOI: 10.7270/Q25Q4XV9 |
More data for this
Ligand-Target Pair | |
Lactate dehydrogenase B (LDHB)
(Homo sapiens (Human)) | BDBM50103568

(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH1 in presence of NADH |
J Med Chem 59: 487-96 (2016)
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50103568

(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
Lactate dehydrogenase B (LDHB)
(Homo sapiens (Human)) | BDBM50103568

(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes LDHB using sodium pyruvate as substrate after 5 mins in presence of NAPDH by diaphorase/resazurin based fluorescence... |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic
(Homo sapiens (Human)) | BDBM50103568

(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of wild type IDH1 (unknown origin) using isocitrate as substrate preincubated for 30 mins followed by substrate addition in presence of NA... |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50103568

(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.18E+3 | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Stabilization of LDHA in human A673 cell lysate preincubated for 20 mins followed by incubation at 70 degreeC for 10 mins by cellular thermal shift a... |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50103568

(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LDH in human A673 cells assessed as reduction in lactate production preincubated for 2 hrs measured after 30 mins by high throughput fl... |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50103568

(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LDH in human MIAPaCa2 cells assessed as reduction in lactate production preincubated for 2 hrs measured after 30 mins by high throughpu... |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50103568

(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human liver LDHA using sodium pyruvate as substrate after 5 mins in presence of NAPDH and EDTA by diaphorase/resazurin based fluorescen... |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50103568

(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human liver LDHA using sodium pyruvate as substrate after 5 mins in presence of NAPDH and in absence of EDTA by diaphorase/resazurin ba... |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50103568

(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged LDHA in absence of NADH by SPR assay |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |
Lactate dehydrogenase A (LDHA)
(Homo sapiens (Human)) | BDBM50103568

(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of LDHA (unknown origin) |
Bioorg Med Chem Lett 24: 4915-25 (2014)
BindingDB Entry DOI: 10.7270/Q25Q4XV9 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50103568

(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged LDHA in presence of NADH by SPR assay |
J Med Chem 60: 9184-9204 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00941
BindingDB Entry DOI: 10.7270/Q2DJ5J28 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data