

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50104301 2-[((R)-1-Hydroxy-ethylamino)-methyl]-pyrrolidine-3,4-diol::BDBM50402987::CHEMBL2206827::CHEMBL79763
SMILES: C[C@H](O)NC[C@H]1NC[C@H](O)[C@@H]1O
InChI Key: InChIKey=ILPLXBQAXZPQTP-BJMCJPPVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-mannosidase (Glycine max) | BDBM50104301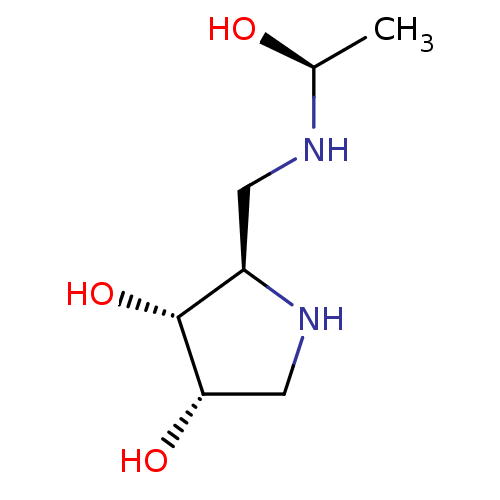 (2-[((R)-1-Hydroxy-ethylamino)-methyl]-pyrrolidine-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Jack bean | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase A (Caldocellum saccharolyticum) | BDBM50104301 (2-[((R)-1-Hydroxy-ethylamino)-methyl]-pyrrolidine-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Beta-Glucosidase from Caldocellum saccharol. | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Prunus avium) | BDBM50104301 (2-[((R)-1-Hydroxy-ethylamino)-methyl]-pyrrolidine-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Beta-Glucosidase from Caldocellum saccharol. | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endoplasmic reticulum mannosyl-oligosaccharide 1,2-alpha-mannosidase (Homo sapiens (Human)) | BDBM50104301 (2-[((R)-1-Hydroxy-ethylamino)-methyl]-pyrrolidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687 Curated by ChEMBL | Assay Description Inhibition of human ER alpha mannosidase 1 | Bioorg Med Chem 20: 6945-59 (2012) Article DOI: 10.1016/j.bmc.2012.10.011 BindingDB Entry DOI: 10.7270/Q26T0NTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Golgi alpha-mannosidase II (Homo sapiens (Human)) | BDBM50104301 (2-[((R)-1-Hydroxy-ethylamino)-methyl]-pyrrolidine-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.83E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade do Porto 687 Curated by ChEMBL | Assay Description Inhibition of human golgi alpha mannosidase 2 | Bioorg Med Chem 20: 6945-59 (2012) Article DOI: 10.1016/j.bmc.2012.10.011 BindingDB Entry DOI: 10.7270/Q26T0NTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Aspergillus niger) | BDBM50104301 (2-[((R)-1-Hydroxy-ethylamino)-methyl]-pyrrolidine-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Beta-galactosidase from Aspergillus niger | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase (Prunus avium) | BDBM50104301 (2-[((R)-1-Hydroxy-ethylamino)-methyl]-pyrrolidine-...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Beta-Glucosidase from Almond | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucosidase A (Caldocellum saccharolyticum) | BDBM50104301 (2-[((R)-1-Hydroxy-ethylamino)-methyl]-pyrrolidine-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Beta-Glucosidase from Caldocellum saccharol. | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase (Glycine max) | BDBM50104301 (2-[((R)-1-Hydroxy-ethylamino)-methyl]-pyrrolidine-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne Curated by ChEMBL | Assay Description Inhibitory activity towards Alpha-mannosidase from Jack bean | Bioorg Med Chem Lett 11: 2489-93 (2001) BindingDB Entry DOI: 10.7270/Q2H70GBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||