

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50104435 4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl ester::CHEMBL87563
SMILES: CCOC(=O)c1ccc(OC(=O)CCCCCNC(N)=N)cc1
InChI Key: InChIKey=YKGYIDJEEQRWQH-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasminogen (Homo sapiens (Human)) | BDBM50104435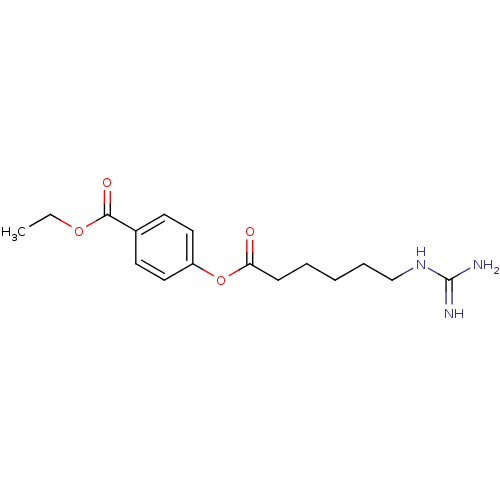 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Competitive inhibition of human plasmin assessed as reduction in hydrolytic activity using S-2251 as substrate by spectrophotometric method | J Med Chem 63: 1445-1472 (2020) Article DOI: 10.1021/acs.jmedchem.9b01060 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | 2.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular adhesion protein 1 (VAP-1) (Rattus norvegicus (Rat)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Technology Curated by ChEMBL | Assay Description Inhibitory activity of the compound against porcine kidney amine oxidase | Bioorg Med Chem Lett 11: 2565-8 (2001) BindingDB Entry DOI: 10.7270/Q25T3JR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepsin (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 383 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Helsinki Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal His10-tagged human Hepsin (R45 to L17 residues) D161E/ R162K double mutant expressed in mouse NS0 cells using Bo... | J Med Chem 61: 4335-4347 (2018) Article DOI: 10.1021/acs.jmedchem.7b01698 BindingDB Entry DOI: 10.7270/Q2FF3VVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 687 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human plasma thrombin using pyroGlu-Pro-Arg-pNA-HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of purified human factor 10a using Suc-Ile-Glu(gammaPip)-GlyArg-pNa-HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using D-Pro-Phe-Arg-pNA-2HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator/surface receptor (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 431 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of recombinant human tPA using H-D-Ile-Pro-L-Arg-pNA-2HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug and toxin extrusion protein 1 (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human MATE1-mediated ASP+ uptake expressed in HEK293 cells after 1.5 mins by fluorescence assay | J Med Chem 56: 781-95 (2013) Article DOI: 10.1021/jm301302s BindingDB Entry DOI: 10.7270/Q2F76DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 2 (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human OCT2-mediated ASP+ uptake expressed in HEK293 cells after 3 mins by fluorescence assay | J Med Chem 56: 781-95 (2013) Article DOI: 10.1021/jm301302s BindingDB Entry DOI: 10.7270/Q2F76DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug and toxin extrusion protein 2 (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human MATE2K-mediated ASP+ uptake expressed in HEK293 cells after 1.5 mins by fluorescence assay | J Med Chem 56: 781-95 (2013) Article DOI: 10.1021/jm301302s BindingDB Entry DOI: 10.7270/Q2F76DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human plasma plasmin using pyroGlu-Pro-Arg-pNA-HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||