

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50104931 (4-{(4-Bromo-phenyl)-[(E)-ethoxyimino]-methyl}-4''-methyl-[1,4'']bipiperidinyl-1''-yl)-(2,4-dimethyl-1-oxy-pyridin-3-yl)-methanone::(4-{(4-Bromo-phenyl)-[(Z)-ethoxyimino]-methyl}-4''''-methyl-[1,4'''']bipiperidinyl-1''''-yl)-(2,4-dimethyl-1-oxy-pyridin-3-yl)-methanone::(4-{(4-Bromo-phenyl)-[(Z)-ethoxyimino]-methyl}-4'-methyl-[1,4']bipiperidinyl-1'-yl)-(2,4-dimethyl-1-oxy-pyridin-3-yl)-methanone::3-(4-((4-bromophenyl)(ethoxyimino)methyl)-4''-methyl-1,4''-bipiperidine-1''-carbonyl)-1,2,4-trimethylpyridinium::CHEMBL336672::CHEMBL78535::SCH-351125::{4-[(4-Bromo-phenyl)-ethoxyimino-methyl]-4''-methyl-[1,4'']bipiperidinyl-1''-yl}-(2,4-dimethyl-1-oxy-pyridin-3-yl)-methanone
SMILES: CCO[N-][C+](C1CCN(CC1)C1(C)CCN(CC1)C(=O)c1c(C)cc[n+]([O-])c1C)c1ccc(Br)cc1
InChI Key: InChIKey=ZGDKVKUWTCGYOA-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Mus musculus) | BDBM50104931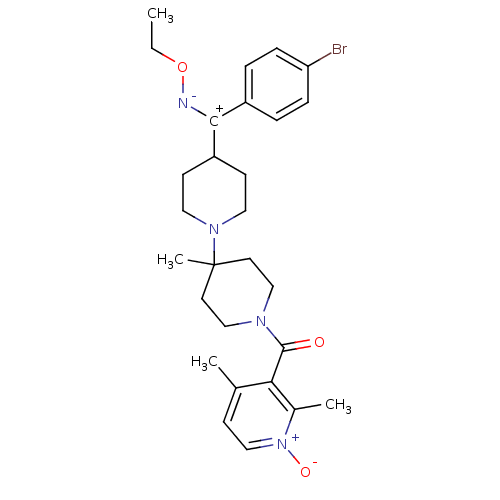 ((4-{(4-Bromo-phenyl)-[(E)-ethoxyimino]-methyl}-4''...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to Chemokine receptor type 5 receptor from NIH 3T3 cells | J Med Chem 45: 3143-60 (2002) BindingDB Entry DOI: 10.7270/Q2HD7TZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104931 ((4-{(4-Bromo-phenyl)-[(E)-ethoxyimino]-methyl}-4''...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsley F. Kimball Research Institute of The New York Blood Center Curated by ChEMBL | Assay Description Inhibition of RANTES binding to the human C-C chemokine receptor type 5 (CCR5) | J Med Chem 46: 4501-15 (2003) Article DOI: 10.1021/jm030265z BindingDB Entry DOI: 10.7270/Q2SB46GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104931 ((4-{(4-Bromo-phenyl)-[(E)-ethoxyimino]-methyl}-4''...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES binding to C-C chemokine receptor type 5 | J Med Chem 44: 3339-42 (2001) BindingDB Entry DOI: 10.7270/Q27M0771 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50104931 ((4-{(4-Bromo-phenyl)-[(E)-ethoxyimino]-methyl}-4''...) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp. Curated by ChEMBL | Assay Description Displacement of [128I]-RANTES from CCR5 expressed in HEK293F cells | Bioorg Med Chem Lett 21: 2450-5 (2011) Article DOI: 10.1016/j.bmcl.2011.02.058 BindingDB Entry DOI: 10.7270/Q2F76CV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||