

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50108862 CHEMBL161651::[1-(1-Benzyl-3-tert-butylsulfanyl-2-oxo-propylcarbamoyl)-2-phenyl-ethyl]-carbamic acid benzyl ester::benzyl (S)-1-((S)-4-(tert-butylthio)-3-oxo-1-phenylbutan-2-ylamino)-1-oxo-3-phenylpropan-2-ylcarbamate
SMILES: CC(C)(C)SCC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1
InChI Key: InChIKey=OXOTXDHSGCISLV-SVBPBHIXSA-N
Data: 4 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Procathepsin L (Homo sapiens (Human)) | BDBM50108862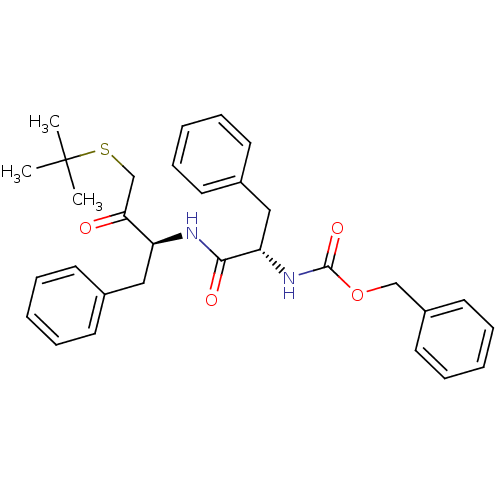 (CHEMBL161651 | [1-(1-Benzyl-3-tert-butylsulfanyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Cathepsin L | J Med Chem 45: 676-84 (2002) BindingDB Entry DOI: 10.7270/Q2ZK5G0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50108862 (CHEMBL161651 | [1-(1-Benzyl-3-tert-butylsulfanyl-2...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against cruzain, the major cysteine protease found in T. cruzi | J Med Chem 45: 676-84 (2002) BindingDB Entry DOI: 10.7270/Q2ZK5G0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cruzipain (Trypanosoma cruzi) | BDBM50108862 (CHEMBL161651 | [1-(1-Benzyl-3-tert-butylsulfanyl-2...) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi cruzain | Bioorg Med Chem 16: 838-53 (2008) Article DOI: 10.1016/j.bmc.2007.10.048 BindingDB Entry DOI: 10.7270/Q2H70GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50108862 (CHEMBL161651 | [1-(1-Benzyl-3-tert-butylsulfanyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity of the compound against human Cathepsin B | J Med Chem 45: 676-84 (2002) BindingDB Entry DOI: 10.7270/Q2ZK5G0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||