

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50109061 3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahydro-pyridin-4-yl}-1H-pyrrolo[2,3-b]pyridine::CHEMBL104229
SMILES: C(CN1CCC(=CC1)c1c[nH]c2ncccc12)Oc1cccc2[nH]ccc12
InChI Key: InChIKey=RTLZRRBLSUHEBN-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1 Adrenergic Receptor/ adrenergic receptor/ adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50109061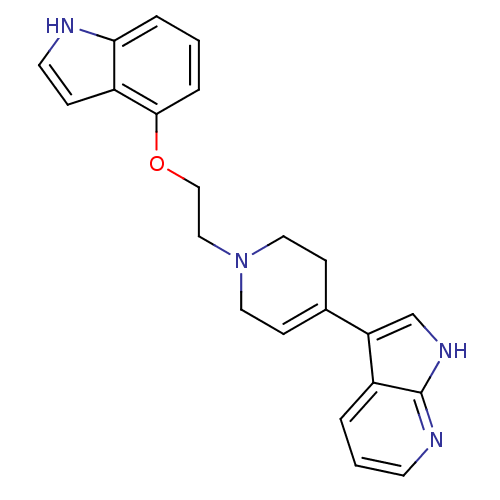 (3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahyd...) | Reactome pathway KEGG DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards Alpha-1 adrenergic receptor was determined by the displacement of [3H]prazosin | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic receptor alpha-1 (Homo sapiens (Human)) | BDBM50109061 (3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahyd...) | MMDB KEGG B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Curated by ChEMBL | Assay Description Inhibition constant against alpha adrenergic receptor | J Med Chem 48: 6523-43 (2005) Article DOI: 10.1021/jm058225d BindingDB Entry DOI: 10.7270/Q2SF2WZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50109061 (3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahyd...) | PDB KEGG DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Curated by ChEMBL | Assay Description Inhibition constant against serotonin transporter | J Med Chem 48: 6523-43 (2005) Article DOI: 10.1021/jm058225d BindingDB Entry DOI: 10.7270/Q2SF2WZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50109061 (3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahyd...) | PDB KEGG GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity of the compound for RB Serotonin transporter was determined in vitro by incubating compound | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50109061 (3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahyd...) | PDB GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Curated by ChEMBL | Assay Description Inhibition constant against 5-hydroxytryptamine 1A receptor | J Med Chem 48: 6523-43 (2005) Article DOI: 10.1021/jm058225d BindingDB Entry DOI: 10.7270/Q2SF2WZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50109061 (3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahyd...) | PDB GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Antagonism at the 5-hydroxytryptamine 1A receptor in vitro | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50109061 (3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahyd...) | PDB KEGG DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Affinity for HC Serotonin transporter determined in vitro by incubating compound and [3H]-5-HT with human carcinoma (Jar cells), previously treated w... | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50109061 (3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahyd...) | PDB GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Antagonism of 5-hydroxytryptamine 1A receptor determined in vitro | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50109061 (3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahyd...) | PDB GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories Curated by ChEMBL | Assay Description Antagonism of 5-hydroxytryptamine 1A receptor was determined in vitro using a [35S]-GTP-gammaS, | Bioorg Med Chem Lett 12: 307-10 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2G2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||