

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50109609 (2R)-2-amino-3-mercaptopropanoic acid::(2R)-2-amino-3-sulfanylpropanoic acid::(R)-2-amino-3-mercaptopropanoic acid::CHEMBL863::CYSTEINE::FREE CYSTEINE::L-Cystein::L-Zystein::L-cysteine
SMILES: N[C@@H](CS)C(O)=O
InChI Key: InChIKey=XUJNEKJLAYXESH-REOHCLBHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cystine/glutamate transporter (Homo sapiens (Human)) | BDBM50109609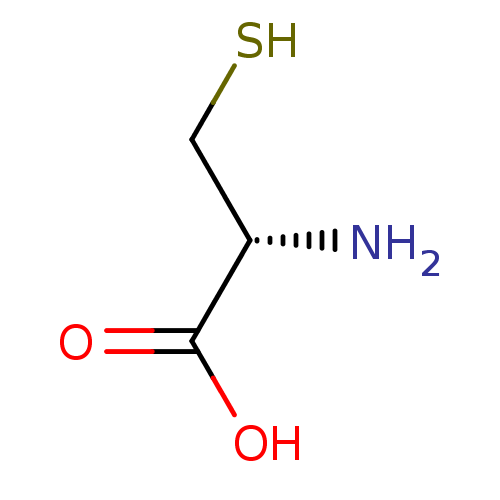 ((2R)-2-amino-3-mercaptopropanoic acid | (2R)-2-ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Competitive inhibition of [3H]L-glutamate uptake at amino acid transport system xc- in human SNB19 cells by liquid scintillation counting | Bioorg Med Chem 18: 202-13 (2010) Article DOI: 10.1016/j.bmc.2009.11.001 BindingDB Entry DOI: 10.7270/Q23J3DX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50109609 ((2R)-2-amino-3-mercaptopropanoic acid | (2R)-2-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pohang University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of bovine carboxypeptidase A (CPA) using hippuryl-L-phenylalanine (Hipp-L-Phe) as a substrate. | J Med Chem 45: 911-8 (2002) BindingDB Entry DOI: 10.7270/Q2VM4BJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proton-coupled amino acid transporter 1 (Homo sapiens (Human)) | BDBM50109609 ((2R)-2-amino-3-mercaptopropanoic acid | (2R)-2-ami...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 4.60E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of human PAT1-mediated L-[3H]proline uptake in human Caco2 cells after 10 mins by liquid scintillation counting | Bioorg Med Chem 19: 6409-18 (2011) Checked by Author Article DOI: 10.1016/j.bmc.2011.08.058 BindingDB Entry DOI: 10.7270/Q2NZ882D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Crotalus adamanteus) | BDBM50109609 ((2R)-2-amino-3-mercaptopropanoic acid | (2R)-2-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 7.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of snake venom phosphodiesterase using bis-(para-nitrophenyl)phosphate as substrate after 30 min by spectrophotometric analysis | Citation and Details Article DOI: 10.1007/s00044-013-0523-6 BindingDB Entry DOI: 10.7270/Q2GH9MV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase (Homo sapiens (Human)) | BDBM50109609 ((2R)-2-amino-3-mercaptopropanoic acid | (2R)-2-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CHINA PHARMACEUTICAL UNIVERSITY US Patent | Assay Description 11.1 Preparation of Reagents and Standard Solutions(1) 75 mM phosphate buffer (PB, pH 7.4): containing KH2PO4 0.0956 g, K2HPO4 0.6946 g, EDTA 1.862 m... | US Patent US11021454 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-like protein 1 (Homo sapiens (Human)) | BDBM50109609 ((2R)-2-amino-3-mercaptopropanoic acid | (2R)-2-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Inhibition of Eg5 assessed as inhibition ATP hydrolysis by ATPase assay | Bioorg Med Chem Lett 17: 3921-4 (2007) Article DOI: 10.1016/j.bmcl.2007.04.101 BindingDB Entry DOI: 10.7270/Q2542N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cystine/glutamate transporter (Homo sapiens (Human)) | BDBM50109609 ((2R)-2-amino-3-mercaptopropanoic acid | (2R)-2-ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Montana Curated by ChEMBL | Assay Description Inhibition of amino acid transport system xc- in human SNB19 cells assessed as [3H]L-glutamate uptake by liquid scintillation counting | Bioorg Med Chem 18: 202-13 (2010) Article DOI: 10.1016/j.bmc.2009.11.001 BindingDB Entry DOI: 10.7270/Q23J3DX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||