

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50109688 3,4,5-Trihydroxy-benzoic acid 5,7-dihydroxy-chroman-3-yl ester::CHEMBL151315
SMILES: Oc1cc(O)c2CC(COc2c1)OC(=O)c1cc(O)c(O)c(O)c1
InChI Key: InChIKey=JHAUIQDZLXLNSY-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50109688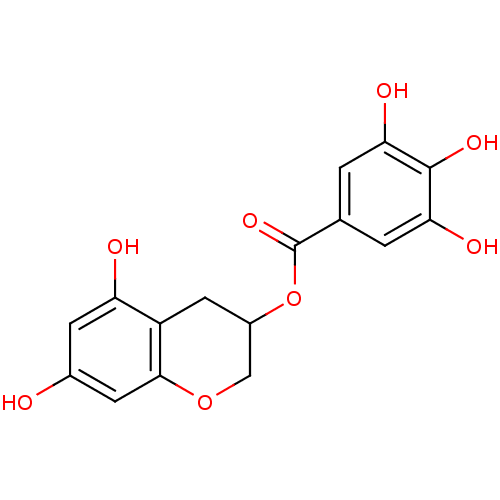 (3,4,5-Trihydroxy-benzoic acid 5,7-dihydroxy-chroma...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase polymerization using A17 double mutant HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50109688 (3,4,5-Trihydroxy-benzoic acid 5,7-dihydroxy-chroma...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase polymerization using wild type HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50109688 (3,4,5-Trihydroxy-benzoic acid 5,7-dihydroxy-chroma...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Tested for its ability to inhibit HIV-1 reverse transcriptase polymerization using A17 double mutant HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50109688 (3,4,5-Trihydroxy-benzoic acid 5,7-dihydroxy-chroma...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase polymerization using wild type HIV-1-RT enzyme | Bioorg Med Chem Lett 12: 525-8 (2002) BindingDB Entry DOI: 10.7270/Q26W99DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||