

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50110600 5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1,1-dimethyl-2-oxo-ethyl]-2-(3,5-dimethyl-phenyl)-1H-indol-3-yl]-propylamino}-ethyl)-1H-benzoimidazole-2-carboxylic acid::CHEMBL352051
SMILES: C[C@H](CNCCc1ccc2nc([nH]c2c1)C(O)=O)c1c([nH]c2ccc(cc12)C(C)(C)C(=O)N1CC2CCC1CC2)-c1cc(C)cc(C)c1
InChI Key: InChIKey=AQWCEAQPXIENDG-WUXOVTSDSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gonadotropin releasing hormone 1 (GnRHR1) (Homo sapiens (Human)) | BDBM50110600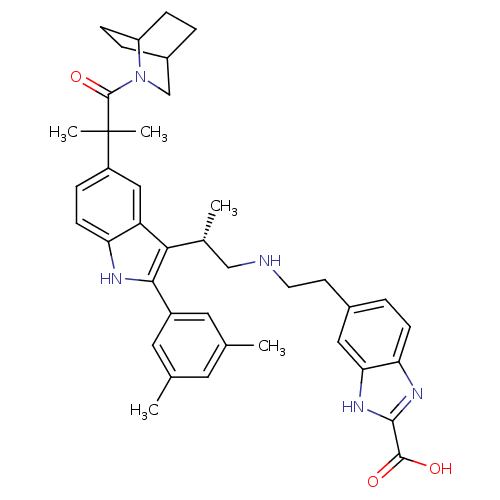 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50110600 (5-(2-{(S)-2-[5-[2-(2-Aza-bicyclo[2.2.2]oct-2-yl)-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of gonadotropin-releasing hormone receptor-stimulated [3H]-inositol phosphate hydrolysis | Bioorg Med Chem Lett 12: 827-32 (2002) BindingDB Entry DOI: 10.7270/Q2G73D1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||