 Found 17 hits for monomerid = 50110681
Found 17 hits for monomerid = 50110681 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine protein phosphatase 2A, 56 kDa regulatory subunit, alpha isoform
(Homo sapiens (Human)) | BDBM50110681
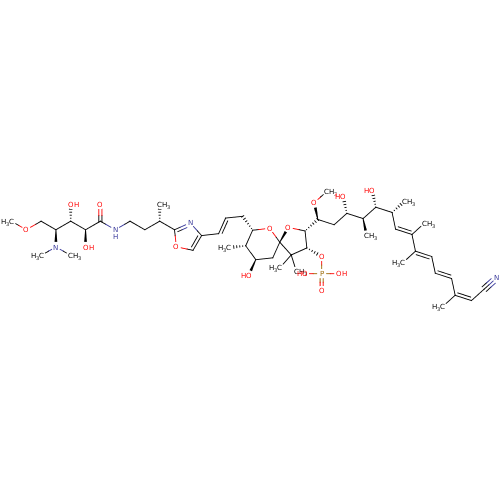
(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of purified catalytic subunit of protein phosphatase-2A from human erythrocytes |
Bioorg Med Chem Lett 3: 1029-1034 (1993)
Article DOI: 10.1016/S0960-894X(00)80281-4
BindingDB Entry DOI: 10.7270/Q2125T5P |
More data for this
Ligand-Target Pair | |
Serine/threonine protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in H248N |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine-threonine protein phosphatase 2A regulatory subunit
(Gallus gallus) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the action of protein phosphatase 2A |
Bioorg Med Chem Lett 12: 391-3 (2002)
BindingDB Entry DOI: 10.7270/Q2XS5VX8 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Protein phosphatase 1 was determined; 0.5-1.0 |
Bioorg Med Chem Lett 12: 391-3 (2002)
BindingDB Entry DOI: 10.7270/Q2XS5VX8 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity of the compounds against protein phosphatases 1 (PP1) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Serine-threonine protein phosphatase 2A regulatory subunit
(Gallus gallus) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Observed inhibition activity of the compounds against protein phosphatases 2A (PP2A) |
J Med Chem 40: 3199-206 (1997)
Article DOI: 10.1021/jm960873x
BindingDB Entry DOI: 10.7270/Q25Q4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in wild type |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in N124D |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in D220V |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in Y272F |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in R221S |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in E275R |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine-threonine protein phosphatase 2A regulatory subunit
(Gallus gallus) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Exogenous inhibition concentration of Serine/threonine protein phosphatase 2A (PP2A) |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | |
Serine/threonine protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in C127S |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in D208A |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine protein phosphatase PP1-gamma catalytic subunit
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle
Curated by ChEMBL
| Assay Description
Inhibition of Serine/threonine protein phosphatase 1 (PP1) mutants by natural toxins in R96A |
J Med Chem 45: 1151-75 (2002)
BindingDB Entry DOI: 10.7270/Q2DJ5GCG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition against purified catalytic subunit of protein phosphatase-1 from rabbit skeletal muscle |
Bioorg Med Chem Lett 3: 1029-1034 (1993)
Article DOI: 10.1016/S0960-894X(00)80281-4
BindingDB Entry DOI: 10.7270/Q2125T5P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data