

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50111589 2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocyclohexa-2,5-dien-1-ylidene)methyl]benzoate::2-[3,5-diiodo-4-olatophenyl(3,5-diiodo-4-oxo-2,5-cyclohexadienyliden)methyl]benzoate
SMILES: [#8-]-[#6](=O)-c1ccccc1\[#6](=[#6]-1\[#6]=[#6](I)-[#6](=O)-[#6](I)=[#6]-1)-c1cc(I)c(-[#8-])c(I)c1
InChI Key: InChIKey=KVFPRLVRGPLMDH-UHFFFAOYSA-L
Data: 15 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase AmpC (Escherichia coli) | BDBM50111589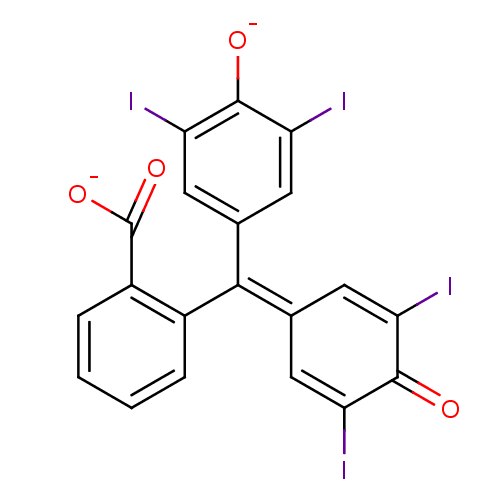 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against beta-lactamase | J Med Chem 45: 1712-22 (2002) BindingDB Entry DOI: 10.7270/Q20C4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (TEM-1) (Escherichia coli) | BDBM50111589 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against TEM-1 Beta-lactamase mutant G238A at 24 degree Centigrade | J Med Chem 46: 4265-72 (2003) Article DOI: 10.1021/jm030266r BindingDB Entry DOI: 10.7270/Q29C6Z59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50111589 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against cloned Dihydrofolate reductase (cDHFR) | J Med Chem 45: 1712-22 (2002) BindingDB Entry DOI: 10.7270/Q20C4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50111589 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of Beta-galactosidase | J Med Chem 45: 1712-22 (2002) BindingDB Entry DOI: 10.7270/Q20C4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase AmpC (Escherichia coli) | BDBM50111589 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against beta-lactamase in the presence of 50 mM KPi concentration of buffer | J Med Chem 45: 1712-22 (2002) BindingDB Entry DOI: 10.7270/Q20C4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase AmpC (Escherichia coli) | BDBM50111589 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against beta-lactamase in the presence of 500 mMKPi concentration of buffer | J Med Chem 45: 1712-22 (2002) BindingDB Entry DOI: 10.7270/Q20C4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin (Homo sapiens (Human)) | BDBM50111589 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against chymotrypsinogen | J Med Chem 45: 1712-22 (2002) BindingDB Entry DOI: 10.7270/Q20C4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-chymotrypsin (Homo sapiens (Human)) | BDBM50111589 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Malate dehydrogenase (Thermus thermophilus) | BDBM50111589 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (TEM-1) (Escherichia coli) | BDBM50111589 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against TEM-1 Beta-lactamase mutant M182T at 42 degree Centigrade | J Med Chem 46: 4265-72 (2003) Article DOI: 10.1021/jm030266r BindingDB Entry DOI: 10.7270/Q29C6Z59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase AmpC (Escherichia coli) | BDBM50111589 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Beta-lactamase from DMSO stock was determined | J Med Chem 46: 4265-72 (2003) Article DOI: 10.1021/jm030266r BindingDB Entry DOI: 10.7270/Q29C6Z59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (TEM-1) (Escherichia coli) | BDBM50111589 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against TEM-1 Beta-lactamase mutant G238A at 42 degree Centigrade | J Med Chem 46: 4265-72 (2003) Article DOI: 10.1021/jm030266r BindingDB Entry DOI: 10.7270/Q29C6Z59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (TEM-1) (Escherichia coli) | BDBM50111589 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against TEM-1 Beta-lactamase mutant M182T at 42 degree Centigrade | J Med Chem 46: 4265-72 (2003) Article DOI: 10.1021/jm030266r BindingDB Entry DOI: 10.7270/Q29C6Z59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase AmpC (Escherichia coli) | BDBM50111589 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Amp C beta-Lactamase | J Med Chem 46: 4265-72 (2003) Article DOI: 10.1021/jm030266r BindingDB Entry DOI: 10.7270/Q29C6Z59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase AmpC (Escherichia coli) | BDBM50111589 (2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against beta-lactamase in the presence of 5 mM KPi concentration of buffer | J Med Chem 45: 1712-22 (2002) BindingDB Entry DOI: 10.7270/Q20C4WHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||