

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50112600 CHEMBL3609392
SMILES: CCS(=O)(=O)c1ccc(CC(=O)Nc2ccc(c(Cl)c2)-c2ccccc2C(F)(F)F)cc1
InChI Key: InChIKey=WYXIDCLTNIMPSC-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM50112600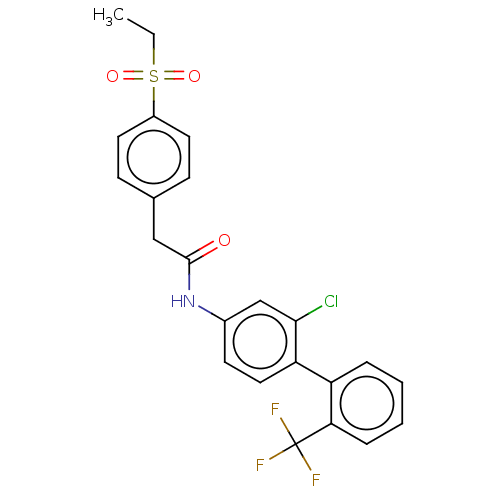 (CHEMBL3609392) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of RORgammat (unknown origin) assessed as inhibition of agonist-induced response by FRET assay | ACS Med Chem Lett 6: 787-92 (2015) BindingDB Entry DOI: 10.7270/Q2XG9SWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM50112600 (CHEMBL3609392) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at RORgammat in mouse CD positive T cells assessed as inhibition of Th17 cell differentiation by measuring 1L-17 release aft... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM50112600 (CHEMBL3609392) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at APC-labeled RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bip... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM50112600 (CHEMBL3609392) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inverse agonist activity at biotinylated RORgammat LBD (unknown origin) assessed as inhibition of N-(2-chloro-6-fluorobenzyl)-N-((2'-methoxy-[1,1'-bi... | ACS Med Chem Lett 9: 120-124 (2018) Article DOI: 10.1021/acsmedchemlett.7b00476 BindingDB Entry DOI: 10.7270/Q2Q81GM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||