

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50117090 (E,E)-Psammaplin A::3-(3-Bromo-4-hydroxy-phenyl)-N-(2-{2-[3-(3-bromo-4-hydroxy-phenyl)-2-hydroxyimino-propionylamino]-ethyldisulfanyl}-ethyl)-2-[(E)-hydroxyimino]-propionamide::3-(3-Bromo-4-hydroxy-phenyl)-N-(2-{2-[3-(3-bromo-4-hydroxy-phenyl)-2-hydroxyimino-propionylamino]-ethyldisulfanyl}-ethyl)-2-hydroxyimino-propionamide::3-(3-Bromo-4-hydroxy-phenyl)-N-[2-(2-{3-(3-bromo-4-hydroxy-phenyl)-2-[(E)-hydroxyimino]-propionylamino}-ethyldisulfanyl)-ethyl]-2-[(E)-hydroxyimino]-propionamide::3-(3-bromo-4-hydroxy-phenyl)-N-[2-(2-{3-(3-bromo-4-hydroxy-phenyl)-2-[hydroxyimino]-propionylamino}-ethyldisulfanyl)-ethyl]-2-[hydroxyimino]-propionamide::CHEMBL83747::Psammaplin A
SMILES: Oc1ccc(CC(N=O)C(=O)NCCSSCCNC(=O)C(Cc2ccc(O)c(Br)c2)N=O)cc1Br
InChI Key: InChIKey=GLBYQIQGWFGGQH-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50117090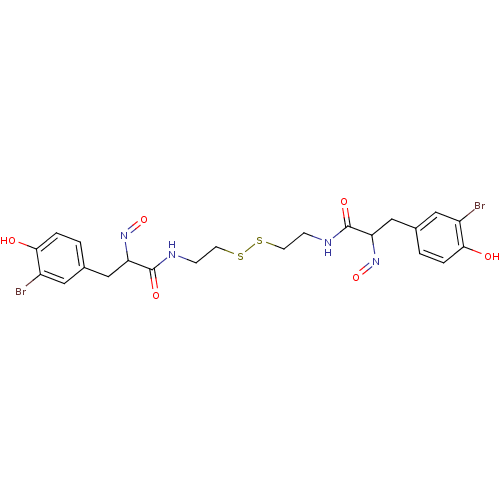 ((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Displacement of [125I]DPCPX from adenosine A1 receptor in rat brain | J Nat Prod 63: 393-5 (2000) BindingDB Entry DOI: 10.7270/Q26H4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mycothiol S-conjugate amidase (Mycobacterium tuberculosis) | BDBM50117090 ((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of Mycothiol -S-conjugate amidase (MCA) from mycobacterium tuberculosis | Bioorg Med Chem Lett 12: 2487-90 (2002) BindingDB Entry DOI: 10.7270/Q2XD111C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor (Homo sapiens (Human)) | BDBM50117090 ((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a |
University of Mississippi Curated by ChEMBL | Assay Description Activation of PPARgamma (unknown origin) transfected in human MCF7 cells by luciferase reporter gene assay | J Nat Prod 69: 547-52 (2006) Article DOI: 10.1021/np050397q BindingDB Entry DOI: 10.7270/Q2P26XWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mycothiol S-conjugate amidase (Mycobacterium tuberculosis) | BDBM50117090 ((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis detoxification enzyme mycothiol-S-conjugate amidase (MCA) | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mycothiol S-conjugate amidase (Mycobacterium tuberculosis) | BDBM50117090 ((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis detoxification enzyme mycothiol-S-conjugate amidase (MCA) | J Med Chem 48: 5613-38 (2005) Article DOI: 10.1021/jm050524f BindingDB Entry DOI: 10.7270/Q2V988V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50117090 ((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cells using Fluor deLys as substrate assessed as deacetylation of substrate by fluorescence assay | Bioorg Med Chem Lett 26: 4372-6 (2016) Article DOI: 10.1016/j.bmcl.2015.12.094 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cereblon/Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50117090 ((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (DNMT1) (Homo sapiens (Human)) | BDBM50117090 ((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of semi-purified DNMT1 | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50117090 ((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50117090 ((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa cells using Fluor deLys as substrate assessed as deacetylation of substrate by fluorescence assay | Bioorg Med Chem Lett 26: 4372-6 (2016) Article DOI: 10.1016/j.bmcl.2015.12.094 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-glucose 4-epimerase (Homo sapiens (Human)) | BDBM50117090 ((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human GalE by HPAEC assay | Bioorg Med Chem Lett 16: 5744-7 (2006) Article DOI: 10.1016/j.bmcl.2006.08.091 BindingDB Entry DOI: 10.7270/Q2RJ4K83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||