

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Cc1cn(C\C=C/COC(c2ccccc2)(c2ccccc2)c2ccccc2)c(=O)[nH]c1=O
InChI Key: InChIKey=BHMZJUVAVWRNLN-QXMHVHEDSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50118490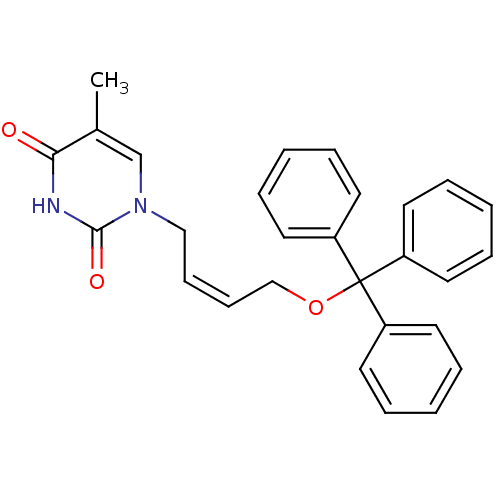 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of TK2 | J Med Chem 49: 7766-73 (2006) Article DOI: 10.1021/jm0610550 BindingDB Entry DOI: 10.7270/Q2K073XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxynucleoside kinase (Drosophila melanogaster) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of [3H]methyl dThd phosphorylation by Drosophila melanogaster dNK | J Med Chem 49: 7766-73 (2006) Article DOI: 10.1021/jm0610550 BindingDB Entry DOI: 10.7270/Q2K073XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of [3H]methyl dThd phosphorylation by TK2 | J Med Chem 49: 7766-73 (2006) Article DOI: 10.1021/jm0610550 BindingDB Entry DOI: 10.7270/Q2K073XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase (Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibition of [3H]methyl dThd phosphorylation by HSV1 TK | J Med Chem 49: 7766-73 (2006) Article DOI: 10.1021/jm0610550 BindingDB Entry DOI: 10.7270/Q2K073XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase 2, mitochondrial (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ghent University Curated by ChEMBL | Assay Description Inhibition of thymidine kinase 2 | J Med Chem 53: 2902-12 (2010) Article DOI: 10.1021/jm901532h BindingDB Entry DOI: 10.7270/Q26D5T47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by human Thymidine Kinase 2 | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with gancicclovir | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration against HSV-1 TK (A167Y) catalyzed [3H]-GCV phosphorylation | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with BVAraU | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration against HSV-1 TK (WT) catalyzed [3H]-GCV phosphorylation | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against phosphorylation of [methyl-3H]dThd by HSV-1 Thymidine kinase | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by HSV-1 Thymidine Kinase | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration of the compound on phosphorylation of [methyl-3H]-dTh by HSV-1 Thymidine Kinase | Bioorg Med Chem Lett 13: 3027-30 (2003) BindingDB Entry DOI: 10.7270/Q2T15310 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||