 Found 12 hits for monomerid = 50118779
Found 12 hits for monomerid = 50118779 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50118779
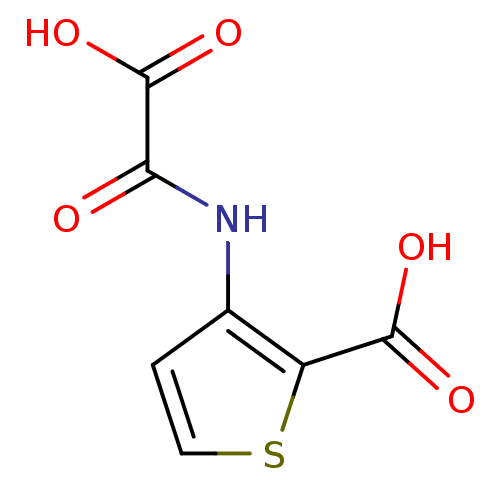
(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50118779

(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsinghua University
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant PTP1B |
Bioorg Med Chem Lett 20: 3329-37 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.033
BindingDB Entry DOI: 10.7270/Q27D2WCC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50118779

(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN2 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase beta (PTPβ)
(Homo sapiens (Human)) | BDBM50118779

(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRB |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Leukocyte common antigen
(Homo sapiens (Human)) | BDBM50118779

(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRC |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase epsilon (PTPε)
(Homo sapiens (Human)) | BDBM50118779

(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRE |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase F (LAR)
(Homo sapiens (Human)) | BDBM50118779

(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRF |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1C
(Homo sapiens (Human)) | BDBM50118779

(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPN6 |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase alpha
(Homo sapiens (Human)) | BDBM50118779

(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Miami
Curated by ChEMBL
| Assay Description
Inhibition of PTPRA |
J Med Chem 52: 6649-59 (2009)
Article DOI: 10.1021/jm9008899
BindingDB Entry DOI: 10.7270/Q29023T5 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase F (LAR)
(Homo sapiens (Human)) | BDBM50118779

(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against protein-tyrosine phosphatase Lar, using p-nitrophenyl phosphate as substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase alpha
(Homo sapiens (Human)) | BDBM50118779

(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibitory effect against human protein-tyrosine phosphatase alpha (PTPalpha), using p-nitrophenyl phosphate substrate at pH 5.5. |
J Med Chem 45: 4443-59 (2002)
BindingDB Entry DOI: 10.7270/Q2QJ7GM5 |
More data for this
Ligand-Target Pair | |
Protein-tyrosine phosphatase 1B
(Homo sapiens (Human)) | BDBM50118779

(3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...)Show InChI InChI=1S/C7H5NO5S/c9-5(7(12)13)8-3-1-2-14-4(3)6(10)11/h1-2H,(H,8,9)(H,10,11)(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70E+13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate University of Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Binding affinity to PTP1B |
Bioorg Med Chem Lett 15: 5521-5 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.078
BindingDB Entry DOI: 10.7270/Q2TF0139 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data