 Found 12 hits for monomerid = 50119132
Found 12 hits for monomerid = 50119132 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50119132
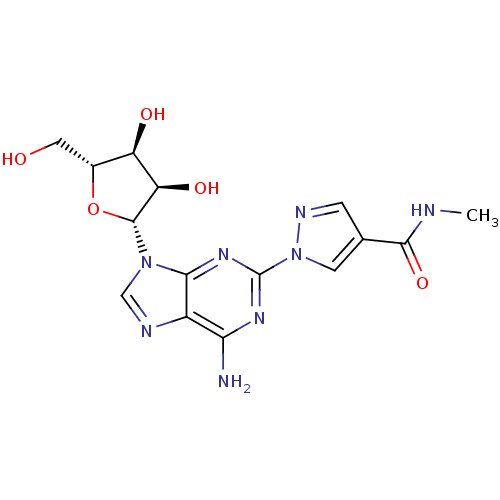
(1-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydrox...)Show SMILES CNC(=O)c1cnn(c1)-c1nc(N)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 Show InChI InChI=1S/C15H18N8O5/c1-17-13(27)6-2-19-23(3-6)15-20-11(16)8-12(21-15)22(5-18-8)14-10(26)9(25)7(4-24)28-14/h2-3,5,7,9-10,14,24-26H,4H2,1H3,(H,17,27)(H2,16,20,21)/t7-,9-,10-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Binding affinity to A2A adenosine receptor |
J Med Chem 55: 538-52 (2012)
Article DOI: 10.1021/jm201461q
BindingDB Entry DOI: 10.7270/Q22Z160R |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50119132

(1-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydrox...)Show SMILES CNC(=O)c1cnn(c1)-c1nc(N)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 Show InChI InChI=1S/C15H18N8O5/c1-17-13(27)6-2-19-23(3-6)15-20-11(16)8-12(21-15)22(5-18-8)14-10(26)9(25)7(4-24)28-14/h2-3,5,7,9-10,14,24-26H,4H2,1H3,(H,17,27)(H2,16,20,21)/t7-,9-,10-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant adenosine receptor A2a |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50119132

(1-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydrox...)Show SMILES CNC(=O)c1cnn(c1)-c1nc(N)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 Show InChI InChI=1S/C15H18N8O5/c1-17-13(27)6-2-19-23(3-6)15-20-11(16)8-12(21-15)22(5-18-8)14-10(26)9(25)7(4-24)28-14/h2-3,5,7,9-10,14,24-26H,4H2,1H3,(H,17,27)(H2,16,20,21)/t7-,9-,10-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Ferrara
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
J Med Chem 58: 3253-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00215
BindingDB Entry DOI: 10.7270/Q2X068RF |
More data for this
Ligand-Target Pair | |
Adenosine Receptors A2a (A2a)
(Rattus norvegicus (rat)) | BDBM50119132

(1-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydrox...)Show SMILES CNC(=O)c1cnn(c1)-c1nc(N)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 Show InChI InChI=1S/C15H18N8O5/c1-17-13(27)6-2-19-23(3-6)15-20-11(16)8-12(21-15)22(5-18-8)14-10(26)9(25)7(4-24)28-14/h2-3,5,7,9-10,14,24-26H,4H2,1H3,(H,17,27)(H2,16,20,21)/t7-,9-,10-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics
Curated by ChEMBL
| Assay Description
Receptor binding affinity for the adenosine A2A receptor was determined using [3H]-ZM-241,385 as a radioligand in rat |
Bioorg Med Chem Lett 12: 2935-9 (2002)
BindingDB Entry DOI: 10.7270/Q2NK3DDV |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50119132

(1-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydrox...)Show SMILES CNC(=O)c1cnn(c1)-c1nc(N)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 Show InChI InChI=1S/C15H18N8O5/c1-17-13(27)6-2-19-23(3-6)15-20-11(16)8-12(21-15)22(5-18-8)14-10(26)9(25)7(4-24)28-14/h2-3,5,7,9-10,14,24-26H,4H2,1H3,(H,17,27)(H2,16,20,21)/t7-,9-,10-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics
Curated by ChEMBL
| Assay Description
Receptor binding affinity for the adenosine A2A receptor were determined using [3H]-ZM-241,385 as a radioligand in pig |
Bioorg Med Chem Lett 12: 2935-9 (2002)
BindingDB Entry DOI: 10.7270/Q2NK3DDV |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50119132

(1-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydrox...)Show SMILES CNC(=O)c1cnn(c1)-c1nc(N)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 Show InChI InChI=1S/C15H18N8O5/c1-17-13(27)6-2-19-23(3-6)15-20-11(16)8-12(21-15)22(5-18-8)14-10(26)9(25)7(4-24)28-14/h2-3,5,7,9-10,14,24-26H,4H2,1H3,(H,17,27)(H2,16,20,21)/t7-,9-,10-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Engineering Research Center for the Emergency Drug
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human adenosine receptor A2A expressed in HEK-293 cell membrane incubated for 2 hrs by radioligand competitive bind... |
Eur J Med Chem 179: 310-324 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.050 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50119132

(1-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydrox...)Show SMILES CNC(=O)c1cnn(c1)-c1nc(N)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 Show InChI InChI=1S/C15H18N8O5/c1-17-13(27)6-2-19-23(3-6)15-20-11(16)8-12(21-15)22(5-18-8)14-10(26)9(25)7(4-24)28-14/h2-3,5,7,9-10,14,24-26H,4H2,1H3,(H,17,27)(H2,16,20,21)/t7-,9-,10-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant adenosine A1 receptor |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50119132

(1-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydrox...)Show SMILES CNC(=O)c1cnn(c1)-c1nc(N)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 Show InChI InChI=1S/C15H18N8O5/c1-17-13(27)6-2-19-23(3-6)15-20-11(16)8-12(21-15)22(5-18-8)14-10(26)9(25)7(4-24)28-14/h2-3,5,7,9-10,14,24-26H,4H2,1H3,(H,17,27)(H2,16,20,21)/t7-,9-,10-,14-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant adenosine A3 receptor |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50119132

(1-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydrox...)Show SMILES CNC(=O)c1cnn(c1)-c1nc(N)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 Show InChI InChI=1S/C15H18N8O5/c1-17-13(27)6-2-19-23(3-6)15-20-11(16)8-12(21-15)22(5-18-8)14-10(26)9(25)7(4-24)28-14/h2-3,5,7,9-10,14,24-26H,4H2,1H3,(H,17,27)(H2,16,20,21)/t7-,9-,10-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Engineering Research Center for the Emergency Drug
Curated by ChEMBL
| Assay Description
Displacement of [3H]CPX from human adenosine receptor A1 expressed in CHO-K1 cell membranes incubated for 2 hrs by radioligand competitive binding an... |
Eur J Med Chem 179: 310-324 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.050 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50119132

(1-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydrox...)Show SMILES CNC(=O)c1cnn(c1)-c1nc(N)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 Show InChI InChI=1S/C15H18N8O5/c1-17-13(27)6-2-19-23(3-6)15-20-11(16)8-12(21-15)22(5-18-8)14-10(26)9(25)7(4-24)28-14/h2-3,5,7,9-10,14,24-26H,4H2,1H3,(H,17,27)(H2,16,20,21)/t7-,9-,10-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 1.27E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for Adenosine A2A receptor in HEK cells |
Bioorg Med Chem Lett 12: 2935-9 (2002)
BindingDB Entry DOI: 10.7270/Q2NK3DDV |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50119132

(1-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydrox...)Show SMILES CNC(=O)c1cnn(c1)-c1nc(N)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 Show InChI InChI=1S/C15H18N8O5/c1-17-13(27)6-2-19-23(3-6)15-20-11(16)8-12(21-15)22(5-18-8)14-10(26)9(25)7(4-24)28-14/h2-3,5,7,9-10,14,24-26H,4H2,1H3,(H,17,27)(H2,16,20,21)/t7-,9-,10-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.65E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics
Curated by ChEMBL
| Assay Description
Binding affinity for adenosine (ADO) A1 receptor in CHO cells |
Bioorg Med Chem Lett 12: 2935-9 (2002)
BindingDB Entry DOI: 10.7270/Q2NK3DDV |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50119132

(1-[6-Amino-9-((2R,3R,4S,5R)-3,4-dihydroxy-5-hydrox...)Show SMILES CNC(=O)c1cnn(c1)-c1nc(N)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1 Show InChI InChI=1S/C15H18N8O5/c1-17-13(27)6-2-19-23(3-6)15-20-11(16)8-12(21-15)22(5-18-8)14-10(26)9(25)7(4-24)28-14/h2-3,5,7,9-10,14,24-26H,4H2,1H3,(H,17,27)(H2,16,20,21)/t7-,9-,10-,14-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant adenosine receptor A2b by cAMP assay |
J Med Chem 57: 3623-50 (2014)
Article DOI: 10.1021/jm4011669
BindingDB Entry DOI: 10.7270/Q28P621J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data