

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50119970 2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-((R)-2-methyl-2,3-dihydro-indol-1-yl)-ethanone::CHEMBL320597
SMILES: C[C@@H]1Cc2ccccc2N1C(=O)CN1CCN(Cc2ccc(C)cc2)CC1
InChI Key: InChIKey=BKDQVRCFIJMWOR-LJQANCHMSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50119970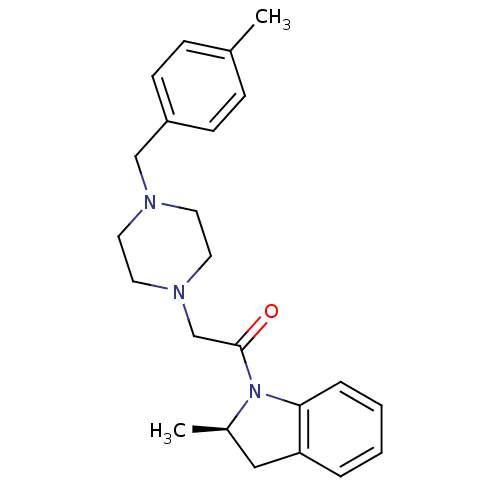 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-((R)-2-me...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy, University of Nebraska Medical Center , Omaha, Nebraska 68198-6125, United States. Curated by ChEMBL | Assay Description Inhibition of human dopamine D4 receptor | J Med Chem 60: 7233-7243 (2017) Article DOI: 10.1021/acs.jmedchem.7b00151 BindingDB Entry DOI: 10.7270/Q2P84FBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50119970 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-((R)-2-me...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D4 was determined via standard competitive displacement assays using [3H]-YM 09151 as the competitive liga... | Bioorg Med Chem Lett 12: 3111-5 (2002) BindingDB Entry DOI: 10.7270/Q2DZ07ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50119970 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-((R)-2-me...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scuola di Scienze del Farmaco e dei Prodotti della Salute , UniversitÓ di Camerino , Via S. Agostino 1 , 62032 Camerino , Italy. Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human dopamine D4 receptor expressed in HEK293 cell membranes after 1 hr by scintillation counting method | J Med Chem 61: 3712-3725 (2018) Article DOI: 10.1021/acs.jmedchem.8b00265 BindingDB Entry DOI: 10.7270/Q20K2C2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50119970 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-((R)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy, University of Nebraska Medical Center , Omaha, Nebraska 68198-6125, United States. Curated by ChEMBL | Assay Description Inhibition of human dopamine D2 receptor | J Med Chem 60: 7233-7243 (2017) Article DOI: 10.1021/acs.jmedchem.7b00151 BindingDB Entry DOI: 10.7270/Q2P84FBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50119970 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-((R)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 was determined via standard competitive displacement assays using [3H]-YM 09151 as the competitive liga... | Bioorg Med Chem Lett 12: 3111-5 (2002) BindingDB Entry DOI: 10.7270/Q2DZ07ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50119970 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-((R)-2-me...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 532 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against the Serotonin transporter | Bioorg Med Chem Lett 12: 3111-5 (2002) BindingDB Entry DOI: 10.7270/Q2DZ07ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptors; mu & delta (Rattus norvegicus (rat)) | BDBM50119970 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-((R)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 976 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against the Opioid receptor delta 1 | Bioorg Med Chem Lett 12: 3111-5 (2002) BindingDB Entry DOI: 10.7270/Q2DZ07ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic receptor alpha-1 (Homo sapiens (Human)) | BDBM50119970 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-((R)-2-me...) | MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy, University of Nebraska Medical Center , Omaha, Nebraska 68198-6125, United States. Curated by ChEMBL | Assay Description Inhibition of alpha1 adrenergic receptor (unknown origin) | J Med Chem 60: 7233-7243 (2017) Article DOI: 10.1021/acs.jmedchem.7b00151 BindingDB Entry DOI: 10.7270/Q2P84FBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1 Adrenergic Receptor/ adrenergic receptor/ adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50119970 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-((R)-2-me...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.12E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor was determined by competitive displacement assays using rat brain homogenate with [3H]prazosin a... | Bioorg Med Chem Lett 12: 3111-5 (2002) BindingDB Entry DOI: 10.7270/Q2DZ07ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50119970 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-((R)-2-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Curated by ChEMBL | Assay Description Inhibition constant against dopamine receptor D2 | J Med Chem 48: 6523-43 (2005) Article DOI: 10.1021/jm058225d BindingDB Entry DOI: 10.7270/Q2SF2WZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adrenergic receptor alpha-1 (Homo sapiens (Human)) | BDBM50119970 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-((R)-2-me...) | MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against alpha adrenergic receptor | J Med Chem 48: 6523-43 (2005) Article DOI: 10.1021/jm058225d BindingDB Entry DOI: 10.7270/Q2SF2WZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50119970 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-((R)-2-me...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against dopamine receptor D4 | J Med Chem 48: 6523-43 (2005) Article DOI: 10.1021/jm058225d BindingDB Entry DOI: 10.7270/Q2SF2WZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||