

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50121317 1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene::4,4'-(2-phenylbut-1-ene-1,1-diyl)diphenol::4-[1-(4-hydroxyphenyl)-2-phenylbut-1-enyl]phenol::CHEMBL149791
SMILES: [#6]-[#6]\[#6](=[#6](\c1ccc(-[#8])cc1)-c1ccc(-[#8])cc1)-c1ccccc1
InChI Key: InChIKey=BPKSDMHGDYTXLI-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM50121317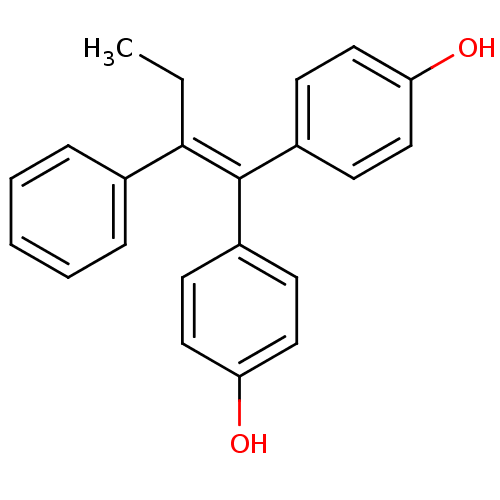 (1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
Georgetown University Curated by ChEMBL | Assay Description Agonist activity at ER in human MCF7:WS8 cells assessed as increase in cell growth by measuring DNA level after 7 days by fluorescence analysis | J Med Chem 57: 4569-83 (2014) Article DOI: 10.1021/jm500569h BindingDB Entry DOI: 10.7270/Q2H996R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50121317 (1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Free University of Berlin Curated by ChEMBL | Assay Description Antiestrogenic activity in MCF-7-2a cells as concentration required to reduce estradiol effect by 50% | J Med Chem 45: 5358-64 (2002) BindingDB Entry DOI: 10.7270/Q2ZK5G19 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM50121317 (1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estradiol receptor beta (ERβ) (Homo sapiens (Human)) | BDBM50121317 (1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 307 | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Displacement of fluorescent ES2 from recombinant human ERbeta after 2 hrs in absence of light by fluorometric analysis | Bioorg Med Chem 24: 5400-5409 (2016) Article DOI: 10.1016/j.bmc.2016.08.064 BindingDB Entry DOI: 10.7270/Q2JQ12ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50121317 (1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | n/a | n/a | 307 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50121317 (1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
the University of Tokyo Curated by ChEMBL | Assay Description Binding affinity to GST-tagged human ER alpha ligand-binding domain by TR-FRET assay | Bioorg Med Chem 27: 1952-1961 (2019) Article DOI: 10.1016/j.bmc.2019.03.042 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50121317 (1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
the University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at FLAG-tagged ERalpha (unknown origin) expressed in HEK293 cells assessed as reduction in E2-induced ER-alpha-mediated transcrip... | Bioorg Med Chem 27: 1952-1961 (2019) Article DOI: 10.1016/j.bmc.2019.03.042 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50121317 (1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
the University of Tokyo Curated by ChEMBL | Assay Description Selective estrogen receptor down-regulator activity at FLAG-tagged ERalpha (unknown origin) expressed in HEK293 cells assessed as induction of ERalph... | Bioorg Med Chem 27: 1952-1961 (2019) Article DOI: 10.1016/j.bmc.2019.03.042 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM50121317 (1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | 2.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 19A1 (Homo sapiens (Human)) | BDBM50121317 (1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of human recombinant microsomal aromatase-mediated 7-methoxy-4-trifluoromethylcoumarin conversion to 7-hydroxytrifluoromethylcoumarin prei... | J Med Chem 56: 4611-8 (2013) Article DOI: 10.1021/jm400364h BindingDB Entry DOI: 10.7270/Q2TX3GR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||