

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50121626 4-(2-{4-[3-(4-Chloro-phenyl)-propylsulfanyl]-6-piperazin-1-yl-[1,3,5]triazin-2-ylamino}-ethyl)-phenol::4-(2-{[4-{[3-(4-CHLOROPHENYL)PROPYL]SULFANYL}-6-(1-PIPERAZINYL)-1,3,5-TRIAZIN-2-YL]AMINO}ETHYL)PHENOL::CHEMBL346455
SMILES: Oc1ccc(CCNc2nc(SCCCc3ccc(Cl)cc3)nc(n2)N2CCNCC2)cc1
InChI Key: InChIKey=AIBKIFHSQQYXLG-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol receptor beta (ERβ) (Homo sapiens (Human)) | BDBM50121626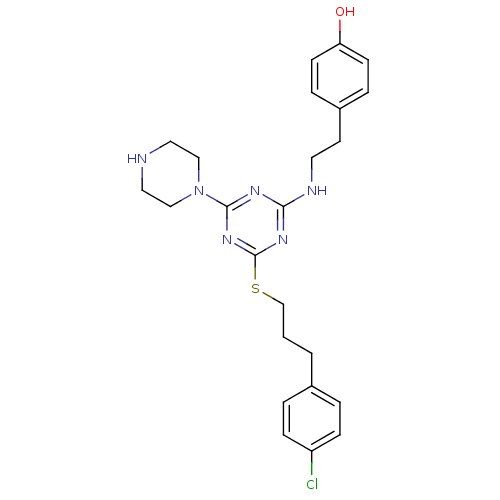 (4-(2-{4-[3-(4-Chloro-phenyl)-propylsulfanyl]-6-pip...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor 2 using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50121626 (4-(2-{4-[3-(4-Chloro-phenyl)-propylsulfanyl]-6-pip...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro binding affinity towards human Estrogen receptor alpha using [3H]-17-beta-estradiol as radioligand | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50121626 (4-(2-{4-[3-(4-Chloro-phenyl)-propylsulfanyl]-6-pip...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of transcriptional activation induced by 1 nM 17-beta estradiol in T47D cells expressing estrogen receptor alpha | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50121626 (4-(2-{4-[3-(4-Chloro-phenyl)-propylsulfanyl]-6-pip...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Agonist effect on transcriptional activation in T47D cells expressing estrogen receptor alpha | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estradiol receptor beta (ERβ) (Homo sapiens (Human)) | BDBM50121626 (4-(2-{4-[3-(4-Chloro-phenyl)-propylsulfanyl]-6-pip...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of 1 nM 17-beta-estradiol induced transcriptional activation in T47D cells expressing estrogen receptor beta | J Med Chem 45: 5492-505 (2002) BindingDB Entry DOI: 10.7270/Q2J965RW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||