

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50121652 CHEMBL3616851
SMILES: [H][C@]12[C@H](C(O)=O)[C@@]1([H])[C@@](N)(C[C@H]2Sc1nnc(N)[nH]1)C(O)=O
InChI Key: InChIKey=PZICKINIOPPAGP-UESLGPDASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50121652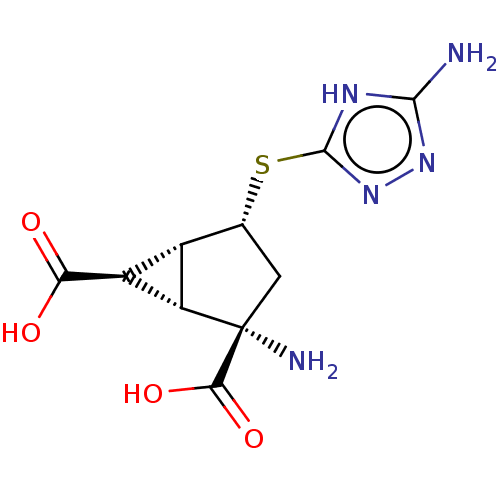 (CHEMBL3616851) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]-459477 from human recombinant mGlu3 receptor expressed in AV12 cells after 90 mins by liquid scintillation counting | J Med Chem 58: 7526-48 (2015) BindingDB Entry DOI: 10.7270/Q29S1ST0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50121652 (CHEMBL3616851) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]-459477 from human recombinant mGlu2 receptor expressed in AV12 cells after 90 mins by liquid scintillation counting | J Med Chem 58: 7526-48 (2015) BindingDB Entry DOI: 10.7270/Q29S1ST0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50121652 (CHEMBL3616851) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 74 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Agonist activity at human recombinant mGlu3 receptor expressed in AV12 cells assessed as inhibition of forskolin-stimulated cAMP production after 1 h... | J Med Chem 58: 7526-48 (2015) BindingDB Entry DOI: 10.7270/Q29S1ST0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50121652 (CHEMBL3616851) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Antagonist activity at human recombinant mGlu3 receptor expressed in AV12 cells assessed as inhibition of glutamate-stimulated Ca2+ mobilization afte... | J Med Chem 58: 7526-48 (2015) BindingDB Entry DOI: 10.7270/Q29S1ST0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50121652 (CHEMBL3616851) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Agonist activity at human recombinant mGlu2 receptor expressed in AV12 cells assessed as inhibition of forskolin-stimulated cAMP production after 1 h... | J Med Chem 58: 7526-48 (2015) BindingDB Entry DOI: 10.7270/Q29S1ST0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50121652 (CHEMBL3616851) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Agonist activity at human recombinant mGlu3 receptor expressed in AV12 cells assessed as stimulation of Ca2+ mobilization after 1.5 hrs by FLIPR assa... | J Med Chem 58: 7526-48 (2015) BindingDB Entry DOI: 10.7270/Q29S1ST0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 2 (Homo sapiens (Human)) | BDBM50121652 (CHEMBL3616851) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Agonist activity at human recombinant mGlu2 receptor expressed in AV12 cells assessed as stimulation of Ca2+ mobilization after 1 hr by FLIPR assay | J Med Chem 58: 7526-48 (2015) BindingDB Entry DOI: 10.7270/Q29S1ST0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||