

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50121970 11,12,13,14,25,26,27,28-octahydrotetrabenzo[c,i,m,s][1,2,11,12,6,7,16,17]tetrathiatetraazacycloicosine-11,14,25,28-tetraone::CHEMBL152850
SMILES: O=C1NNC(=O)c2ccccc2SSc2ccccc2C(=O)NNC(=O)c2ccccc2SSc2ccccc12
InChI Key: InChIKey=ZFFCQXUXSGUXST-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50121970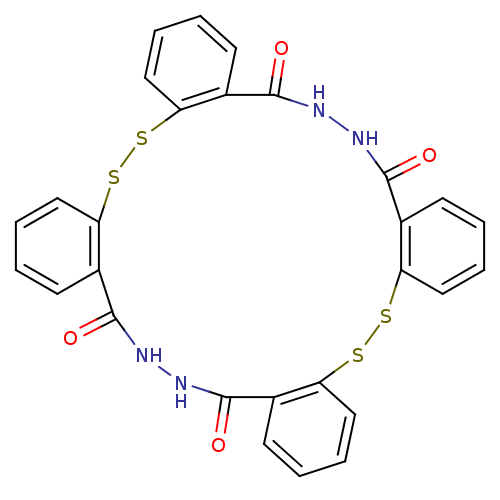 (11,12,13,14,25,26,27,28-octahydrotetrabenzo[c,i,m,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 integrase (preincubated with Mg+2, and DNA on ice for 15 min followed by drug for 1h) in 3'' processing of postasse... | J Med Chem 45: 5661-70 (2002) BindingDB Entry DOI: 10.7270/Q2M61JMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50121970 (11,12,13,14,25,26,27,28-octahydrotetrabenzo[c,i,m,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 integrase (preincubated with Mg+2, and drug for 30 min followed by DNA for 1h) in 3''-processing of preassemble | J Med Chem 45: 5661-70 (2002) BindingDB Entry DOI: 10.7270/Q2M61JMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50121970 (11,12,13,14,25,26,27,28-octahydrotetrabenzo[c,i,m,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 integrase (preincubated with Mg+2, and DNA on ice for 15 min followed by drug for 1h) in strand transfer of postass... | J Med Chem 45: 5661-70 (2002) BindingDB Entry DOI: 10.7270/Q2M61JMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50121970 (11,12,13,14,25,26,27,28-octahydrotetrabenzo[c,i,m,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 integrase (preincubated with Mg+2, and drug for 30 min followed by DNA for 1h) in strand transfer of preassemble | J Med Chem 45: 5661-70 (2002) BindingDB Entry DOI: 10.7270/Q2M61JMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50121970 (11,12,13,14,25,26,27,28-octahydrotetrabenzo[c,i,m,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory activity of the compound against RAdT of HIV-1 Reverse transcriptase | J Med Chem 45: 5661-70 (2002) BindingDB Entry DOI: 10.7270/Q2M61JMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50121970 (11,12,13,14,25,26,27,28-octahydrotetrabenzo[c,i,m,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory activity against rCdG of HIV-1 Reverse transcriptase | J Med Chem 45: 5661-70 (2002) BindingDB Entry DOI: 10.7270/Q2M61JMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50121970 (11,12,13,14,25,26,27,28-octahydrotetrabenzo[c,i,m,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory activity against viral protease | J Med Chem 45: 5661-70 (2002) BindingDB Entry DOI: 10.7270/Q2M61JMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 reverse transcriptase (Human immunodeficiency virus 1) | BDBM50121970 (11,12,13,14,25,26,27,28-octahydrotetrabenzo[c,i,m,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Effective concentration of the compound against viral replication by Reverse transcriptase | J Med Chem 45: 5661-70 (2002) BindingDB Entry DOI: 10.7270/Q2M61JMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50121970 (11,12,13,14,25,26,27,28-octahydrotetrabenzo[c,i,m,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 integrase (preincubated with Mn+2, and DNA on ice for 15 min followed by drug for 1h) in 3'' processing of postasse... | J Med Chem 45: 5661-70 (2002) BindingDB Entry DOI: 10.7270/Q2M61JMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50121970 (11,12,13,14,25,26,27,28-octahydrotetrabenzo[c,i,m,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 integrase (preincubated with Mn+2, and drug for 30 min followed by DNA for 1h) in 3''-processing of preassemble | J Med Chem 45: 5661-70 (2002) BindingDB Entry DOI: 10.7270/Q2M61JMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50121970 (11,12,13,14,25,26,27,28-octahydrotetrabenzo[c,i,m,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 integrase (preincubated with Mn+2, and DNA on ice for 15 min followed by drug for 1h) in strand transfer of postass... | J Med Chem 45: 5661-70 (2002) BindingDB Entry DOI: 10.7270/Q2M61JMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 integrase (Human immunodeficiency virus 1) | BDBM50121970 (11,12,13,14,25,26,27,28-octahydrotetrabenzo[c,i,m,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 integrase (preincubated with Mn+2, and drug for 30 min followed by DNA for 1h) in strand transfer of preassemble | J Med Chem 45: 5661-70 (2002) BindingDB Entry DOI: 10.7270/Q2M61JMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Human immunodeficiency virus type 1 protease (Human immunodeficiency virus type 1) | BDBM50121970 (11,12,13,14,25,26,27,28-octahydrotetrabenzo[c,i,m,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southern California Curated by ChEMBL | Assay Description Inhibitory activity of the compound against viral protease | J Med Chem 45: 5661-70 (2002) BindingDB Entry DOI: 10.7270/Q2M61JMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||