

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50121977 2-(3,4-Dimethoxy-phenyl)-5-[2-(3,4-dimethoxy-phenyl)-ethylamino]-2-isopropyl-pentanenitrile::CHEMBL1298::Norverapamil (6%)
SMILES: COc1ccc(CCNCCCC(C#N)(C(C)C)c2ccc(OC)c(OC)c2)cc1OC
InChI Key: InChIKey=UPKQNCPKPOLASS-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50121977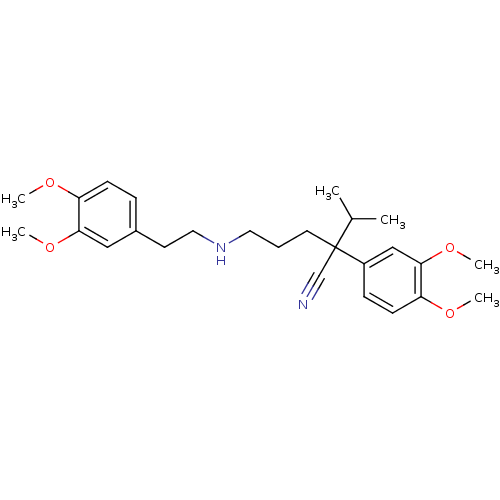 (2-(3,4-Dimethoxy-phenyl)-5-[2-(3,4-dimethoxy-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone 6-beta hydroxylation using human liver microsomes | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (CYP3A5) (Homo sapiens (Human)) | BDBM50121977 (2-(3,4-Dimethoxy-phenyl)-5-[2-(3,4-dimethoxy-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 3A5 measured by testosterone hydroxylation | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50121977 (2-(3,4-Dimethoxy-phenyl)-5-[2-(3,4-dimethoxy-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone 6-beta hydroxylation using a recombinant system | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50121977 (2-(3,4-Dimethoxy-phenyl)-5-[2-(3,4-dimethoxy-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Mechanism based inhibition of human cytochrome P450 3A4 using expressed CYP3A4 cDNA | Curr Drug Metab 6: 413-54 (2005) BindingDB Entry DOI: 10.7270/Q2VQ33X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM50121977 (2-(3,4-Dimethoxy-phenyl)-5-[2-(3,4-dimethoxy-pheny...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bulgarian Academy of Sciences Curated by ChEMBL | Assay Description Concentration required for 50% inhibition at binding site of human P-Glycoprotein (P-gp) in one-affinity model | J Med Chem 45: 5671-86 (2002) BindingDB Entry DOI: 10.7270/Q23B60VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM50121977 (2-(3,4-Dimethoxy-phenyl)-5-[2-(3,4-dimethoxy-pheny...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Digoxin transepithelial transport (basal to apical) (Digoxin: 5 uM) in Caco-2 cells | J Pharmacol Exp Ther 293: 376-82 (2000) BindingDB Entry DOI: 10.7270/Q2RB75VT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P-glycoprotein 1 (Homo sapiens (Human)) | BDBM50121977 (2-(3,4-Dimethoxy-phenyl)-5-[2-(3,4-dimethoxy-pheny...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Daunorubicin efflux in NIH-3T3-G185 cells | Chem Res Toxicol 14: 1596-603 (2001) BindingDB Entry DOI: 10.7270/Q20P119B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||