

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50122102 3-Cyano-4-hydroxy-benzoic acid [1-(2,3,5,6-tetramethyl-benzyl)-1H-indol-4-ylmethylene]-hydrazide::CHEMBL152640::N'-((1-(2,3,5,6-tetramethylbenzyl)-1H-indol-4-yl)methylene)-3-cyano-4-hydroxybenzohydrazide
SMILES: Cc1cc(C)c(C)c(Cn2ccc3c(\C=N\NC(=O)c4ccc(O)c(c4)C#N)cccc23)c1C
InChI Key: InChIKey=HNTXQDRFWJBZGO-FJEPWZHXSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pituitary adenylate cyclase-activating polypeptide type I receptor (Homo sapiens (Human)) | BDBM50122102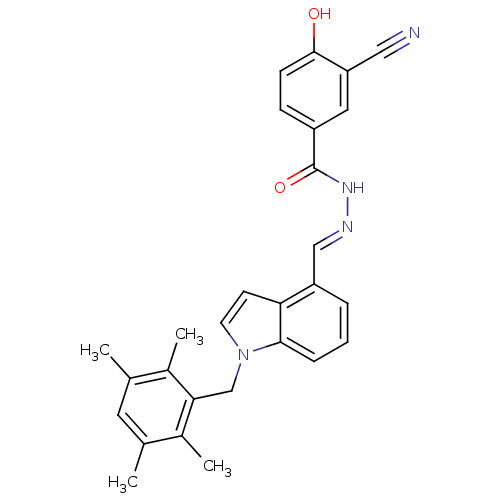 (3-Cyano-4-hydroxy-benzoic acid [1-(2,3,5,6-tetrame...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]PACAP27 from PAC1R expressed in HEK293f cells | Bioorg Med Chem Lett 18: 2162-6 (2008) Article DOI: 10.1016/j.bmcl.2008.01.052 BindingDB Entry DOI: 10.7270/Q22J6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50122102 (3-Cyano-4-hydroxy-benzoic acid [1-(2,3,5,6-tetrame...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity against human glucagon receptor | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50122102 (3-Cyano-4-hydroxy-benzoic acid [1-(2,3,5,6-tetrame...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description In vitro binding affinity of the compound towards rat glucagon receptor | J Med Chem 45: 5755-75 (2002) BindingDB Entry DOI: 10.7270/Q26W99FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50122102 (3-Cyano-4-hydroxy-benzoic acid [1-(2,3,5,6-tetrame...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cells | J Med Chem 45: 5755-75 (2002) BindingDB Entry DOI: 10.7270/Q26W99FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||