

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50123580 CHEMBL3622208
SMILES: [H][C@@]1(CNC(=O)C1)[C@@H](C)Oc1nc(cc2ncsc12)-c1ccc(OC)c(OC)c1
InChI Key: InChIKey=OTUKQYFHRKKGEI-DGCLKSJQSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50123580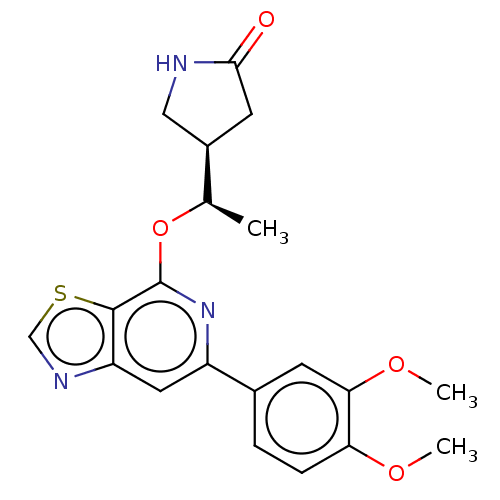 (CHEMBL3622208) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of Syk (unknown origin) using 5-Fluo-Ahx-GAPDYENLQELNKK-Amide as substrate after 60 mins by microfluidic mobility shift assay | Bioorg Med Chem Lett 25: 4642-7 (2015) BindingDB Entry DOI: 10.7270/Q2XD13HG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50123580 (CHEMBL3622208) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of Syk in anti-igM stimulated human Ramos B cells assessed as phospho-BLNK level preincubated for 30 mins followed by anti-IgM stimulation... | Bioorg Med Chem Lett 25: 4642-7 (2015) BindingDB Entry DOI: 10.7270/Q2XD13HG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM50123580 (CHEMBL3622208) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 517 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of Syk in anti-CD32 stimulated CD14+ human monocytes in presence of 90% human blood assessed as phospho-SLP76 level preincubated for 30 mi... | Bioorg Med Chem Lett 25: 4642-7 (2015) BindingDB Entry DOI: 10.7270/Q2XD13HG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||