

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50123658 3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-yl)-(3-hydroxy-phenyl)-methyl]-N-(3-fluoro-phenyl)-N-methyl-benzamide::CHEMBL155892
SMILES: C[C@@H]1CN([C@@H](c2cccc(O)c2)c2cccc(c2)C(=O)N(C)c2cccc(F)c2)[C@@H](C)CN1CC=C
InChI Key: InChIKey=LZXRQLIIMYJZDA-UETOGOEVSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123658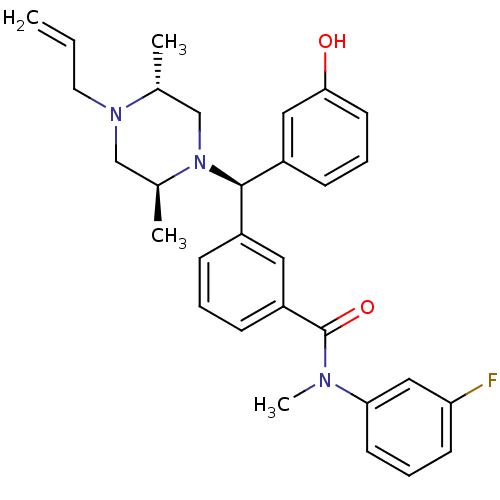 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from mu opioid receptor in albino Sprague-Dawley rat after 90 mins by liquid scintillation | Eur J Med Chem 108: 211-28 (2016) BindingDB Entry DOI: 10.7270/Q2R2137W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123658 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123658 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from kappa opioid receptor in albino Sprague-Dawley rat after 90 mins by liquid scintillation | Eur J Med Chem 108: 211-28 (2016) BindingDB Entry DOI: 10.7270/Q2R2137W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123658 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123658 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor mu 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50123658 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 11.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity of compound evaluated for Opioid receptor delta 1 isolated from rat brain | J Med Chem 46: 623-33 (2003) Article DOI: 10.1021/jm020395s BindingDB Entry DOI: 10.7270/Q2DZ0915 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123658 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor in CD1 mouse assessed as electrically induced twitches | Eur J Med Chem 108: 211-28 (2016) BindingDB Entry DOI: 10.7270/Q2R2137W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50123658 (3-[(R)-((2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-y...) | UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Catania Curated by ChEMBL | Assay Description Agonist activity at mu-opioid receptor in guinea pig ileum assessed as inhibition of electrically induced twitches | Eur J Med Chem 108: 211-28 (2016) BindingDB Entry DOI: 10.7270/Q2R2137W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||