

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50126761 (S)-2-AMINO-3-(3-HYDROXY-5-TERT-BUTYLISOTHIAZOL-4-YL) PROPRIONIC ACID::2-Amino-3-(5-tert-butyl-3-hydroxy-isothiazol-4-yl)-propionic acid::CHEMBL29024
SMILES: CC(C)(C)c1s[nH]c(=O)c1C[C@H](N)C(O)=O
InChI Key: InChIKey=FHWOAQCPEFTDOQ-LURJTMIESA-N
Data: 7 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic kainate 2 (Xenopus laevis) | BDBM50126761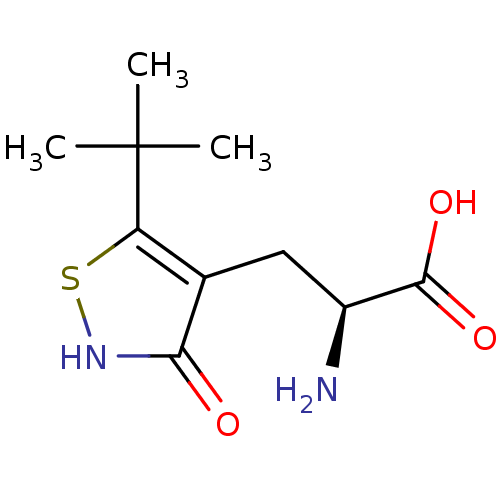 ((S)-2-AMINO-3-(3-HYDROXY-5-TERT-BUTYLISOTHIAZOL-4-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Electrophysiological data on Xenopus oocytes, expressing heteromeric Ionotropic glutamate receptor ionotropic kainate 2 | J Med Chem 46: 1350-8 (2003) Article DOI: 10.1021/jm0204441 BindingDB Entry DOI: 10.7270/Q2902346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic AMPA (Homo sapiens (Human)) | BDBM50126761 ((S)-2-AMINO-3-(3-HYDROXY-5-TERT-BUTYLISOTHIAZOL-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | n/a | n/a | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Electrophysiological data on Xenopus oocytes, expressing homomeric Ionotropic glutamate receptor AMPA 3 (GluR3o) | J Med Chem 46: 1350-8 (2003) Article DOI: 10.1021/jm0204441 BindingDB Entry DOI: 10.7270/Q2902346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Grik5 (Homo sapiens (Human)) | BDBM50126761 ((S)-2-AMINO-3-(3-HYDROXY-5-TERT-BUTYLISOTHIAZOL-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Electrophysiological data on Xenopus oocytes, expressing homomeric kainate receptor (GluR5) | J Med Chem 46: 1350-8 (2003) Article DOI: 10.1021/jm0204441 BindingDB Entry DOI: 10.7270/Q2902346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GRIA1 (Homo sapiens (Human)) | BDBM50126761 ((S)-2-AMINO-3-(3-HYDROXY-5-TERT-BUTYLISOTHIAZOL-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GluA1 (unknown origin) | J Med Chem 56: 593-624 (2013) Article DOI: 10.1021/jm3011433 BindingDB Entry DOI: 10.7270/Q23B61GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GRIA1 (Homo sapiens (Human)) | BDBM50126761 ((S)-2-AMINO-3-(3-HYDROXY-5-TERT-BUTYLISOTHIAZOL-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Electrophysiological data on Xenopus oocytes, expressing homomeric Ionotropic glutamate receptor AMPA 1 (GluR1o) | J Med Chem 46: 1350-8 (2003) Article DOI: 10.1021/jm0204441 BindingDB Entry DOI: 10.7270/Q2902346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GRIK1 (Homo sapiens (Human)) | BDBM50126761 ((S)-2-AMINO-3-(3-HYDROXY-5-TERT-BUTYLISOTHIAZOL-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GluK1 (unknown origin) | J Med Chem 56: 593-624 (2013) Article DOI: 10.1021/jm3011433 BindingDB Entry DOI: 10.7270/Q23B61GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic AMPA (Homo sapiens (Human)) | BDBM50126761 ((S)-2-AMINO-3-(3-HYDROXY-5-TERT-BUTYLISOTHIAZOL-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Electrophysiological data on Xenopus oocytes, expressing homomeric Ionotropic glutamate receptor AMPA 3 (GluR4o) | J Med Chem 46: 1350-8 (2003) Article DOI: 10.1021/jm0204441 BindingDB Entry DOI: 10.7270/Q2902346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||