

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50126829 (E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl)-5,7-dihydroxy-2,2-dimethyl-2H-chromen-8-yl)-3-phenylprop-2-en-1-one::1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl)-5,7-dihydroxy-2,2-dimethyl-2H-chromen-8-yl)-3-phenylprop-2-en-1-one::1-[6-(3-Acetyl-2,4,6-trihydroxy-5-methyl-benzyl)-5,7-dihydroxy-2,2-dimethyl-2H-chromen-8-yl]-3-phenyl-propenone::CHEMBL34241::R5648 (Rottlerin)::ROTTLERIN
SMILES: CC(=O)c1c(O)c(C)c(O)c(Cc2c(O)c3C=CC(C)(C)Oc3c(C(=O)C=Cc3ccccc3)c2O)c1O
InChI Key: InChIKey=DEZFNHCVIZBHBI-UHFFFAOYSA-N
Data: 11 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TGM2/ cRaf Kinase (Homo sapiens (Human)) | BDBM50126829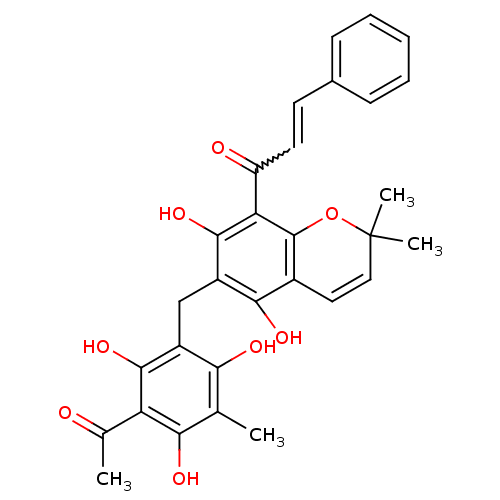 ((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
Duke University Medical Center | Assay Description In order to eliminated fluorescence interference, chemicals were subjected to a secondary screening using colorimetric BP incorporation assay. TGase... | Chem Biol 15: 969-78 (2008) Article DOI: 10.1016/j.chembiol.2008.07.015 BindingDB Entry DOI: 10.7270/Q2DR2SZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 5 (Homo sapiens (Human)) | BDBM50126829 ((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of His-tagged human PRAK expressed in Sf9 cells | Biochem J 351: 95-105 (2001) BindingDB Entry DOI: 10.7270/Q24T6JKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 2 (Homo sapiens (Human)) | BDBM50126829 ((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of His-tagged human MAPKAPK2 expressed in Escherichia coli at 10 uM | Biochem J 351: 95-105 (2001) BindingDB Entry DOI: 10.7270/Q24T6JKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-chymotrypsin (Homo sapiens (Human)) | BDBM50126829 ((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Chymotrypsinogen from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Malate dehydrogenase (Thermus thermophilus) | BDBM50126829 ((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavus | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50126829 ((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae beta-lactamase incubated for 10 mins followed by nitrocefin substrate challenge and measured for 5 mins by spectro... | ACS Med Chem Lett 10: 923-928 (2019) Article DOI: 10.1021/acsmedchemlett.9b00093 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50126829 ((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of Protein kinase C delta (PKCdelta) | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase AmpC (Escherichia coli) | BDBM50126829 ((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibitory activity against Amp C beta-Lactamase | J Med Chem 46: 4265-72 (2003) Article DOI: 10.1021/jm030266r BindingDB Entry DOI: 10.7270/Q29C6Z59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 1/WD repeat-containing protein 48 (Homo sapiens (Human)) | BDBM50126829 ((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Health Center Curated by ChEMBL | Assay Description Inhibition of human USP1/UAF1 complex using Ub-Rho as substrate by qHTS assay | J Med Chem 59: 9321-9336 (2016) BindingDB Entry DOI: 10.7270/Q2ZC84TJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50126829 ((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyushu University Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae beta-lactamase incubated for 10 mins followed by nitrocefin substrate challenge and measured for 5 mins in presenc... | ACS Med Chem Lett 10: 923-928 (2019) Article DOI: 10.1021/acsmedchemlett.9b00093 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-activated protein kinase 5 (Homo sapiens (Human)) | BDBM50126829 ((E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of p38-regulated activated kinase (PRAK) | J Med Chem 46: 1478-83 (2003) Article DOI: 10.1021/jm020427b BindingDB Entry DOI: 10.7270/Q2R78FZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||