

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50128131 (R)-3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(4-chloro-3-ethylphenoxy)phenyl)amino)-1,1,1-trifluoropropan-2-ol::(R)-3-((3-(4-chloro-3-ethylphenoxy)phenyl)(3-(1,1,2,2-tetrafluoroethoxy)benzyl)amino)-1,1,1-trifluoropropan-2-ol::3-{[3-(4-Chloro-3-ethyl-phenoxy)-phenyl]-[3-(1,1,2,2-tetrafluoro-ethoxy)-benzyl]-amino}-1,1,1-trifluoro-propan-2-ol::CHEMBL67129::SC-795
SMILES: CCc1cc(Oc2cccc(c2)N(C[C@@H](O)C(F)(F)F)Cc2cccc(OC(F)(F)C(F)F)c2)ccc1Cl
InChI Key: InChIKey=VHSPKQAESIGBIC-HSZRJFAPSA-N
Data: 6 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50128131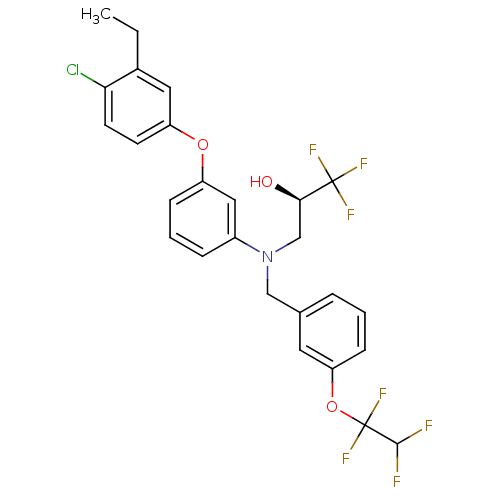 ((R)-3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development) Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in hamster serum | J Med Chem 46: 2152-68 (2003) Article DOI: 10.1021/jm020528+ BindingDB Entry DOI: 10.7270/Q2QV3KWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50128131 ((R)-3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development) Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer | J Med Chem 46: 2152-68 (2003) Article DOI: 10.1021/jm020528+ BindingDB Entry DOI: 10.7270/Q2QV3KWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Oryctolagus cuniculus) | BDBM50128131 ((R)-3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(4-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Zaytoonah Private University of Jordan Curated by ChEMBL | Assay Description Inhibition of CETP in rabbit serum after 1 hr by fluorescent cholesteryl esters transfer assay | Eur J Med Chem 45: 1598-617 (2010) Article DOI: 10.1016/j.ejmech.2009.12.070 BindingDB Entry DOI: 10.7270/Q2VQ32VS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50128131 ((R)-3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development) Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in human serum | J Med Chem 46: 2152-68 (2003) Article DOI: 10.1021/jm020528+ BindingDB Entry DOI: 10.7270/Q2QV3KWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50128131 ((R)-3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development) Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer with <1 nM [CETP] for 18 ... | J Med Chem 46: 2152-68 (2003) Article DOI: 10.1021/jm020528+ BindingDB Entry DOI: 10.7270/Q2QV3KWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50128131 ((R)-3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development) Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in rabbit serum | J Med Chem 46: 2152-68 (2003) Article DOI: 10.1021/jm020528+ BindingDB Entry DOI: 10.7270/Q2QV3KWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||